1. Investigation of Electrolyte Decomposition Byproducts inGas and Liquid Phases Due to Water Impurities in Large-Scale Acetonitrile-Based Supercapacitors
Jiraporn Phojaroen, Phatsawit Wuamprakhon, Thitiphum Sangsanit, Kanruthai Santiyuk, Kan Homlamai, Nichakarn Anansuksawat, Worapol Tejangkura, Ronnachai Songthan, and Montree Sawangphruk
Batteries & Supercaps 2024, e202400738
DOI : 10.1002/batt.202400738
Abstract : This study investigates the impact of water impurities onelectrolyte decomposition in large-scale cylindrical supercapaci-tors, with a focus on acetonitrile-based electrolytes. Theresearch identified ethylene, ethane, and nitrogen as primarygaseous byproducts and acetamide, N-ethyl acetamide, andtrimethylsilyl acetamide as major liquid-phase decompositionproducts. Advanced analytical techniques, including in-situ gaschromatography and nuclear magnetic resonance, revealedthat water impurities significantly accelerate electrolyte degra-dation. The findings demonstrate that water-induced decom-position mechanisms involve intricate pathways, including Hofmann elimination and hydrolysis reactions. Additionally, thepresence of water catalyzes the formation of new byproducts,impacting both the electrolyte and electrode stability. Thiscomprehensive analysis provides critical insights into thedegradation processes of supercapacitors, emphasizing theneed for stringent control of water content to enhance devicelongevity and performance. The study’s outcomes suggestpotential strategies for optimizing electrolyte compositions andelectrode materials to mitigate degradation and improve super-capacitor efficiency.

2. Preconcentration of Lithium Salt in Nanoporous Alumina on Cu foil as Concentrated Lithium Semi-Solid Layer for Anode-free Limetal Batteries
Nichakarn Anansuksawat Thitiphum Sangsanit, Surat Prempluem, Kan Homlamai, Poramane Chiochan, Ronnachai Songthan, Worapol Tejangkura and Montree Sawangphruk
Chemical Communications, 2024.
DOI : 10.1039/D4CC03946G
Abstract : We preconcentrate 2 M LiTFSI in 1-Ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide inside nanoporous alumina (Al2O3) layer so-called concentrated lithium semi-solid layer on Cu current collector for anode-free Li-metal NMC90 batteries. This concept lowers the activation energy for lithium-ion transport and reduces nucleation overpotential, enhancing cycling stability.
3. Designing electrolytes for enhancing stability and performance of lithium-ion capacitors at large-scale cylindrical cells
Phatsawit Wuamprakhon, Ronnachai Songthan, Thitiphum Sangsanit, Kanruthai Santiyuk, Jiraporn Phojaroen, Kan Homlamai, Worapol Tejangkura, Montree Sawangphruk
Journal of Power Sources, 2024, 622, 235331
DOI : https://doi.org/10.1016/j.jpowsour.2024.235331
Abstract : This study investigates the impact of fluorinated ethylene carbonate (FEC) as a co-solvent in the electrolytes of large-scale lithium-ion capacitors (LICs), an area previously unexplored. Our comprehensive analysis integrates electrochemical analysis, gas detection, and surface chemical examination to assess the performance and stability of a 1.2 M LiPF6 electrolyte system. We explored various formulations, including a standard EC/EMC/DEC mixed solvent and newly designed electrolytes with FEC additions, such as EC/EMC/DEC/FEC and DMC/FEC blends. Our findings demonstrate that the DMC/FEC blend matches and even surpasses the capacity retention of traditional electrolytes, suggesting enhanced long-term stability. FEC's presence significantly reduces electrolyte decomposition, as confirmed by in situ Differential Electrochemical Mass Spectrometry and ex-situ gas chromatography. Further, surface chemical analysis highlighted the formation of a stable, LiF-rich solid electrolyte interface (SEI) when FEC is included, thereby improving the chemical stability of the system. These results underline FEC's crucial role in optimizing electrolyte compositions, thus advancing the development of more efficient and durable LIC systems. FEC's integration into LIC electrolytes represents a significant breakthrough, enhancing overall performance and longevity, with broad implications for the application of LICs in various technologies.
4. Gas evolution analyses of Ni-rich Li-ion and Li-metal batteries at cylindrical jelly-roll configurations
Kan Homlamai, Nichakarn Anansuksawat, Thitiphum Sangsanit, Surat Prempluem, Kanruthai Santisuk, Worapol Tejangkura, Montree Sawangphruk
Journal of Power Sources, 2024, 617, 235150
DOI : https://doi.org/10.1016/j.jpowsour.2024.235150
Abstract : Understanding gas evolution during battery operation is pivotal for elucidating the underlying mechanisms of battery behavior, a cornerstone for fostering innovation in energy storage technologies. Despite the wealth of quantitative analyses available, the realm of large-scale, practical, full-cell investigations remains relatively unexplored. This study bridges this gap by unveiling a novel, robust gas chromatography methodology designed to meticulously quantify gas generation within 18650 cylindrical cells Li-ion and anode-free Li-metal Ni-rich batteries across a spectrum of operational conditions. Our approach not only reaffirms the capability for precise, reliable quantitative measurements but also illuminates new findings on the dynamics of gas evolution. This includes the significant impact of temperature and cathode material composition on gas generation, alongside a pioneering exploration into the behavior of anode-free Li-metal batteries. These insights not only advance our understanding of battery performance and safety at a practical cell level but also pave the way for the development of more efficient, durable, and safer energy storage solutions.

5. Carbon Corrosion in Supercapacitors (Corrosion and Degradation in Fuel Cells, Supercapacitors and Batteries)
Montree Sawangphruk
Chapter, 13 June 2024, 179–191
DOI : https://link.springer.com/chapter/10.1007/978-3-031-57012-4_8
Abstract : In this chapter, we delve into carbon corrosion in supercapacitors, a critical challenge impacting their durability and efficiency. Highlighting the degradation of carbon electrodes at high voltages, especially at the positive-electrode side, we explore the factors contributing to this corrosion, including electrolyte interactions and operational conditions. The chapter outlines innovative mitigation strategies, focusing on advanced carbon materials and electrolyte modifications. This comprehensive analysis aims to enhance the understanding of carbon corrosion mechanisms, contributing significantly to the development of more resilient and efficient supercapacitors for sustainable energy technologies.

6. Optimizing Electrode Efficiency in Lithium Titanate: Investigating the Impact of Electrolyte Additives on Cylindrical Li-ion Capacitor Cells
Chalita Aphirakaramwong, Phatsawit Wuamprakhon, Thitiphum Sangsanit, Worapol Tejangkura, Ronnachai Songthan, Montree Sawangphruk
Energy Technology, 16 June 2024
DOI : https://doi.org/10.1002/ente.202301661
Abstract : Recent advancements in lithium-based energy storage focus on new electrode materials for lithium-ion batteries (LIBs) and capacitors. Lithium titanate (LTO) emerges as a key player, offering minimal volume change, rapid charging, and enhanced safety. A critical discovery is the formation of a solid electrolyte interphase (SEI) layer on LTO electrodes, especially with electrolyte additives, improving electrochemical performance. This study investigates additive effects on LTO. Large-scale Li-ion capacitors (LIC) of LTO//activated carbon (AC) are crafted and evaluated. Notably, adding just 1% of methylene methanedisulfonate (MMDS) or fluoroethylene carbonate (FEC) to a 1 m LiPF6 electrolyte markedly increases capacities and maintains performance over 500 cycles, outperforming other additives. These additives also boost large-scale capacitor capacities, showing exceptional stability for over 1600 cycles. This underlines the potential of customized electrolyte additives in elevating LTO-based energy storage, leading to more durable and efficient lithium-based solutions.

7. Selective Conversion of Carbon Dioxide to Formate Using Few-Layer Nitrogen-Doped Graphene on Copper Foam with Enhanced Suppression of Hydrogen Evolution Reaction
Thanthita Sasipatworakarn, Daranphop Pikulrat, Kan Homlamai, Salatan Duangdangchotea and Montree Sawangphruk
Sustainable Energy Fuels, 2024, Advance Article
DOI : https://doi.org/10.1039/D4SE00550C
Abstract : This research presents a novel electrocatalyst, a three-dimensional, few-layered nitrogen-doped graphene-coated copper foam (N-GP/Cu-foam), engineered for the selective electrochemical reduction of carbon dioxide (CO2) to formate. Formate serves as a versatile hydrogen carrier and carbon source, pivotal for subsequent conversion processes into hydrocarbons and oxygenates. Synthesized via chemical vapor deposition, this catalyst markedly suppresses the hydrogen evolution reaction (HER), a predominant competitive reaction in electrochemical CO2 reduction processes. The N-GP/Cu-foam electrocatalyst exhibits a faradaic efficiency of 66.5% for formate production at an overpotential of −1.0 V versus the reversible hydrogen electrode (RHE), demonstrating exceptional selectivity and efficiency. This enhanced performance is attributed primarily to the stabilization of HCOOH* intermediates through the interaction with electron-rich nitrogen dopants embedded within the graphene matrix. Complementary Density Functional Theory (DFT) calculations have further elucidated that the catalyst exhibits a significantly lower Gibbs free energy for HCOOH* compared to CO*, underscoring a strong thermodynamic favorability towards formate production over other potential products. Moreover, the N-GP/Cu-foam catalyst achieves a more than two-fold reduction in the undesired HER, significantly enhancing the overall energy efficiency of the CO2 electrochemical reduction system. These findings underscore the potential of nitrogen-doped graphene to significantly enhance catalytic selectivity and efficiency, offering insightful contributions to the advancement of CO2 utilization technologies. This study provides foundational insights for the future development of more efficient catalytic systems for environmental remediation and sustainable chemical synthesis.

8. Initial formaldehyde generation as a predictive marker for long-term stability of Ni-rich Li-ion batteries under abusive conditions
Thitiphum Sangsanit, Kanruthai Santiyuk, Ronnachai Songthana, Kan Homlamai, Surat Prempluem, Worapol Tejangkura, Montree Sawangphruk
Journal of Power Sources,2024, 611, 234770
DOI : org/10.1016/j.jpowsour.2024.234770
Abstract : Electrolyte decomposition significantly impacts the long-term stability of Li-ion batteries, generating liquid and gaseous byproducts. This study focuses on formaldehyde formation, a critical byproduct from CO2 reduction in Ni-rich Li-ion batteries. Through experimental and computational analyses, formaldehyde emerges from carbonate-based electrolyte decomposition. Examining overcharge, over-discharge, and fast charging conditions, formaldehyde concentration sharply increases under overcharge, indicating severe electrolyte breakdown, while over-discharge shows a gradual rise. Fast charging similarly elevates formaldehyde levels, underscoring the link between electrolyte decomposition and abusive conditions. Importantly, this research proposes utilizing formaldehyde concentration from the initial cycle as a predictive marker for assessing long-term Ni-rich Li-ion battery stability trends. Elevated initial formaldehyde indicates potential capacity fade and accelerated degradation, enabling proactive mitigation strategies. The study elucidates mechanisms governing formaldehyde formation, providing a foundation for developing electrolyte formulations and electrode materials with enhanced decomposition resistance and improved stability. This investigation contributes to the fundamental understanding of electrolyte decomposition in Ni-rich Li-ion batteries and presents a novel approach to predicting long-term performance based on initial formaldehyde measurements, advancing high-performance and reliable energy storage solutions.
9. Revisiting the Effect of Natural and Artificial Graphite on the Performance of Ni-rich Li-ion Batteries at Coin and Cylindrical Cells
Ronnachai Songthan, Thitiphum Sangsanit, Kanruthai Santiyuk, Kan Homlamai, Worapol Tejangkura, and Montree Sawangphruk
Journal of The Electrochemical Society, 2024, 171, 050524
DOI : 10.1149/1945-7111/ad47d8
Abstract : We conducted a detailed evaluation of the electrochemical performance of artificial graphite (AG) and natural graphite (NG) from four leading global companies: AG-1, AG-2, AG-3, and NG-4 towards Ni-rich Li-ion batteries. We found that AG-2, an artificial graphite variant, demonstrated superior performance with exceptional capacity, rapid charging capabilities, and impressive capacity retention. AG-2 achieved a specific capacity of 338.97 mAh g−1, outperforming AG-1 (321.16 mAh g−1), AG-3 (314.43 mAh g−1), and NG-1 (328.08 mAh g−1). This superiority was further confirmed by high C-rate tests ranging from 2 C to 5 C. Notably, after 500 cycles, AG-2 maintained 91.18% of its initial capacity, significantly surpassing AG-1 (89.44%), AG-3 (78.78%), and NG-1 (84.16%). The study attributes AG-2's exceptional performance to its refined properties such as smaller particle size, fewer graphite imperfections, and a higher 2H phase content. These characteristics lead to increased active material in the anode, enhancing battery capacity, and to less material degradation over time, ensuring consistent capacity retention. Overall, AG-2 stands out as a highly efficient and cost-effective option for lithium-ion battery applications, eclipsing other commercial graphite alternatives.

10. Complex reaction mechanisms of electrolyte decomposition at large-scale Ni-rich Li-ion battery cells including electrode crosstalk effect
Surat Prempluem, Thitiphum Sangsanit, Kanruthai Santiyuk, Kan Homlamai, Worapol Tejangkura, Ronnachai Songthan, Nichakarn Anansuksawat, Montree Sawangphruk
Journal of Power Sources, 2024, 606, 234538
DOI : https://doi.org/10.1016/j.jpowsour.2024.234538
Abstract : Ni-rich cathodes are essential for the advancement of high-energy lithium-ion batteries but encounter significant challenges at elevated voltages, including oxygen liberation and enhanced surface reactivity, which precipitate electrolyte decomposition. This study explores the intricate reaction mechanisms of electrolyte decomposition linked to the pronounced release of oxygen from the cathode lattice in large-scale 18650 cylindrical cells employing Ni-rich LiNi0.90Mn0.05Co0.05O2(NMC90)//graphite configurations, focusing on electrode crosstalk effects at high voltages. Notably, acetals such as methoxy methanol (49.93 %) and methane diol (50.07 %) were identified at 4.7 V, with their presence escalating at 4.9 V. Formaldehyde, present at 7.21 %, along with methanol (22.77 %), methane diol (36.35 %), and methoxy methanol (33.67 %), begins to manifest at 4.9 V. The dissolution of transition metals was analyzed using Inductively Coupled Plasma Atomic Emission Spectrometry, and changes in the double-layer capacitance were assessed through electrochemical impedance spectroscopy, further validating the link between these decomposition products and cathode failure associated with oxygen release. These insights reveal the complex relationship between electrolyte decomposition and cathode degradation due to oxygen release, highlighting a critical failure mechanism in large-scale cylindrical lithium-ion batteries.

11. Unveiling a Novel Decomposition Pathway in Propylene Car-bonate-Based Supercapacitors: Insights from a Jelly Roll Config-uration Study
Phatsawit Wuamprakhona, Jiraporn Phojaroena, Thitiphum Sangsanita, Kanruthai Santiyuka, Kan Hom-lamaia, Worapol Tejangkuraa,and Montree Sawangphruka
ChemSusChem, 2024, N/N
DOI : https://doi.org/10.1002/cssc.202400053
Abstract : This research elucidates novel insights into the electrochemical properties and degradation phenomena of propylene carbonate (PC)-based supercapacitors at a large-scale 18650 cylindrical jelly-roll cell level. Central to our findings is the identification of 2-ethyl-4-methyl-1,3-dioxolane (EMD) as a hitherto undocumented decomposition by-product, highlighting the nuanced complexity of PC electrolyte stability. We further demonstrate that elevated operational voltages precipitate accelerated electrolyte degradation, underscoring the criticality of defining the operational voltage window for maximizing device longevity. Employing advanced analytical techniques, including gas chromatography-mass spectrometry (GC-MS), this study meticulously analyzes electrolyte decomposition mechanisms. The outcomes offer pivotal insights into the operational constraints and chemical resilience of PC-based supercapacitors, contributing significantly to the optimization of supercapacitor design and application. By delineating a specific decomposition pathway, this investigation enriches the understanding of electrochemical dynamics in supercapacitor systems, providing a foundation for future research and technological advancement in energy storage devices.

12. How Uniform Particle Size of NMC90 Boosts Li-ion Mobility for Faster Charging and Discharging at a Cylindrical Li-ion Battery Cell
Nichakarn Anansuksawat, Thitiphum Sangsanit, Surat Prempluem, Kan Homlamai, Worapol Tejangkura, and Montree Sawangphruk
Chemical Science, 2024, 2024,15, 2026-2036
DOI : https://doi.org/10.1039/D3SC05698H
Abstract : The pursuit of high-performing cathode materials for next-generation lithium ion batteries has focused on increasing the nickel content of the material. However, this study reveals that particle size uniformity is a more decisive factor in battery performance than the nickel content alone. Using in operando X-ray diffraction, in situ gas evolution, and Atlung intercalant diffusion, we compared two promising cathode materials: LiNi0.8Mn0.1Co0.1O2 (NMC80) and LiNi0.9Mn0.05Co0.05O2 (NMC90). We found that the NMC90 cell, with its more uniform particle size, exhibits a remarkable accumulation energy density of 558 kW h kgNMC−1, which is 22% higher than that of the NMC80 cell. Also, the NMC90 cell unexpectedly has a 20% better capacity retention, a 50-fold higher discharge capacity at the 5C rate, and an Atlung lithium diffusion coefficient that is one order of magnitude higher after 1000 cycles. In situ gas analysis at a high voltage of 4.5 V reveals that the NMC80 cell generates 1.75 times more CO2 than the NMC90 cell. These findings illuminate the intricate relationship between the nickel content, particle size uniformity, and battery performance. They offer vital insights for optimizing cathode materials in future lithium ion batteries.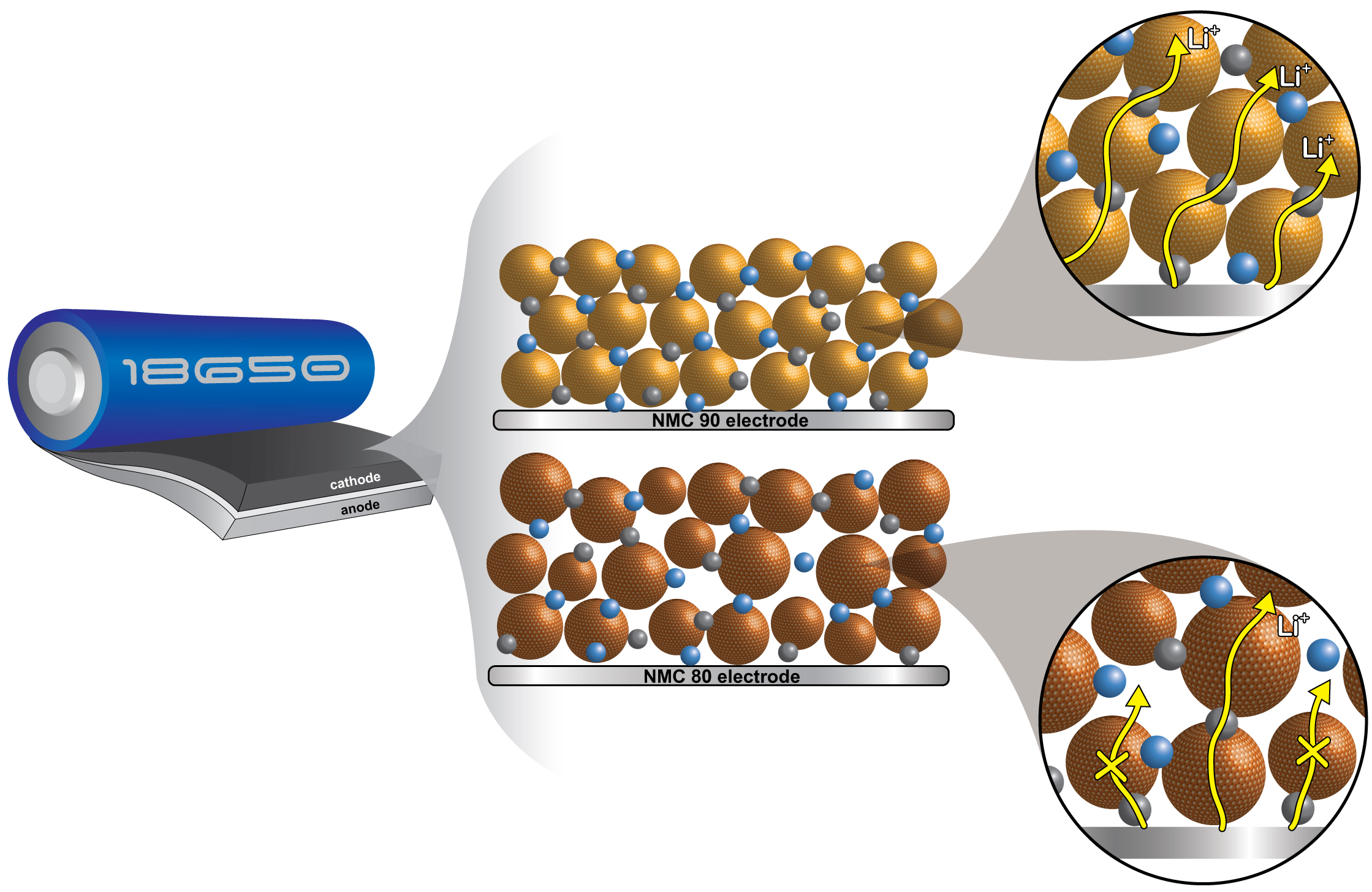

13. Non-flammable electrolyte for large-scale Ni-rich Li-ion batteries: Reducing thermal runaway risks
Thitiphum Sangsanit, Kan Homlamai, Nattanon Joraleechanchai, Surat Prempluem, Worapol Tejangkura, Montree Sawangphruk
Journal of Power Sources, 2024, 594, 234021
DOI : https://doi.org/10.1016/j.jpowsour.2023.234021
Abstract : We present a novel, non-flammable electrolyte for Ni-rich 18650 cylindrical lithium-ion batteries (LIBs) based on a blend of triethyl phosphate (TEP) and fluorinated ethylene carbonate (FEC). We conducted a comprehensive analysis of the electrolyte, including electrochemical tests, safety evaluations, electrode surface characterizations, and quantum calculations, to elucidate its property-function relationship. We found that combining TEP with a concentrated FEC as a co-solvent (1.2 M LiPF6 in TEP/FEC, 30/70 %v) resulted in a robust solid electrolyte interface (SEI) predominantly composed of lithium fluoride (LiF). This innovation improved the SEI's stability and suppressed electrolyte decomposition during extended cycling, leading to consistent battery performance. Our findings introduce a promising non-flammable electrolyte solution for LIBs that offers both thermal runaway prevention and seamless integration into existing battery manufacturing processes. This positions it as a potential alternative to solid-state electrolytes in advanced battery technology, with the potential to revolutionize the field.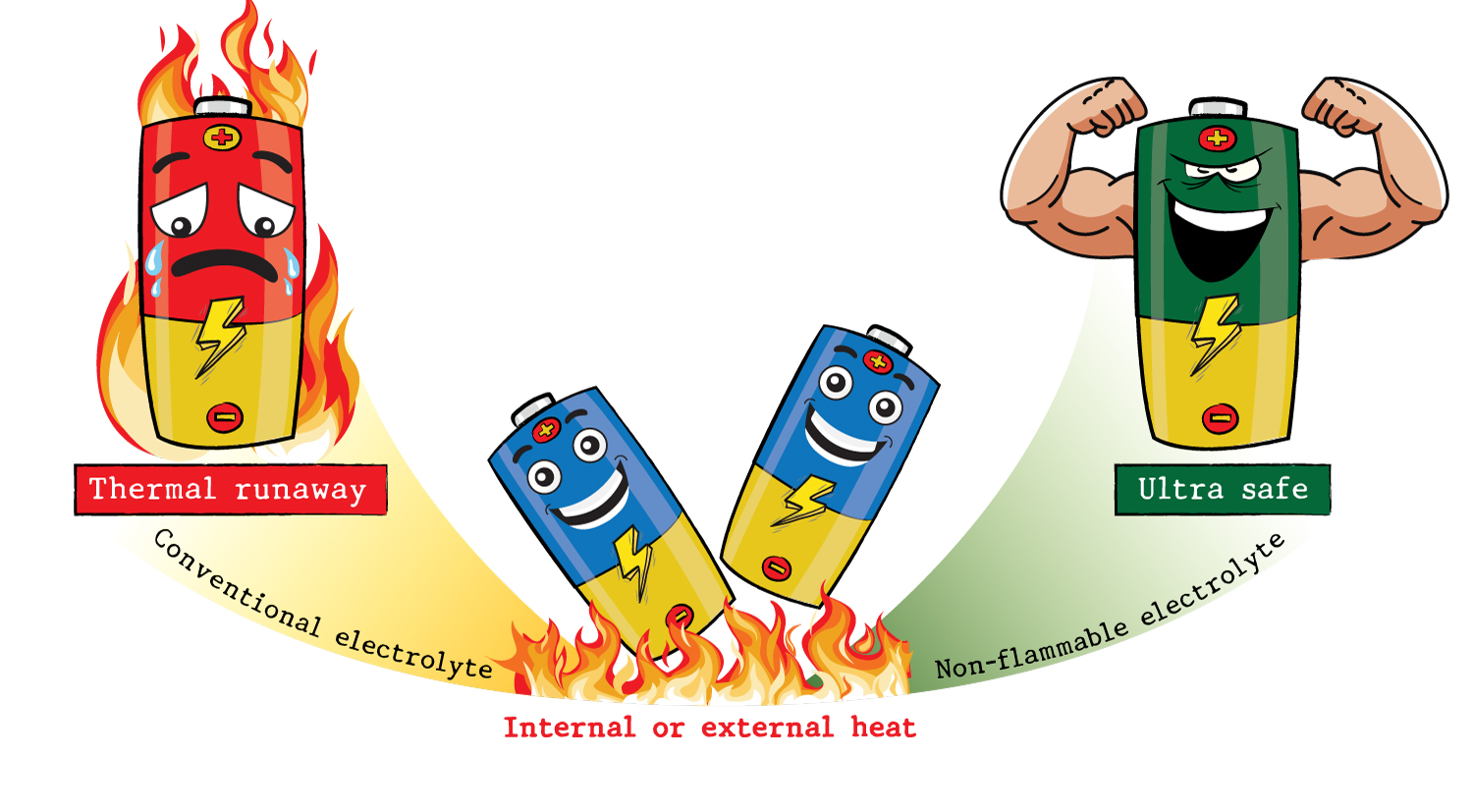

14. Rocking chair-type aqueous sodium-ion capacitors with biomass-derived activated carbon and Na3V2 (PO4) 2F3 nanoflower in a water-in-salt electrolyte
Arisa Phukhrongthung, Montree Sawangphruk, Pawin Iamprasertkun, Charuayporn Santhaweesuk, Channarong Puchongkawarin, Santamon Luanwuthi
Journal of Energy Storage, 2024, 80, 110369
DOI : https://doi.org/10.1016/j.est.2023.110369
Abstract : Sodium-ion capacitors (SICs) are promising energy storage devices that combine the characteristics of high-energy batteries and high-power capacitors, utilising abundant and cost-effective sodium resources. However, conventional SICs employ flammable and toxic organic electrolytes and an electrolyte-consuming mechanism. This work introduces a novel rocking chair-type aqueous sodium-ion capacitor (RC-ASIC) that employs a water-in-salt electrolyte (17 m NaClO4). It incorporates oil palm leaf-derived activated carbon (OPL_AC) as the anode and Na3(VPO4)2F3 with a flower-like structure (NVPF_NF) as the cathode. This RC-ASIC demonstrates a significant improvement in specific energy by extending the voltage window to 2.2 V. Furthermore, the cell with rocking-chair construction requires minimal electrolyte because sodium ions deintercalate from the cathode and adsorb onto the anode surface during charging, thus increasing energy density similarly to rechargeable batteries. The OPL_AC//NVPF_NF cell delivers a high specific energy of 326 Wh/kg at a specific power of 5729 W/kg. Even at an increased specific power of 115 kW/kg, the energy density remains at 31 Wh/kg (based on the total mass of active materials in both electrodes). Consequently, with their remarkable performance, RC-ASICs in a superconcentrated electrolyte system present a promising strategy for advancing energy storage devices.

