1. Impact of Cationic Molecular Length of Ionic Liquid Electrolytes on Cell Performances of 18650 Supercapacitors
Phatsawit Wuamprakhon, Ruttiyakorn Donthongkwa, Kanit Hantanasirisakul, Vinich Promarak, Jumras Limtrakul, Montree Sawangphruk
Chemical Communications, 2021, 57, 13712-13715
DOI: https://pubs.rsc.org/en/content/articlelanding/2021/cc/d1cc05773a#!
Abstract: The specific cell capacitance, equivalent series resistance (ESR) and equivalent distributed resistance (EDR) of porous carbon-based supercapacitors linearly depend on the cation molecular length (1 dimension) of room-temperature ionic liquids.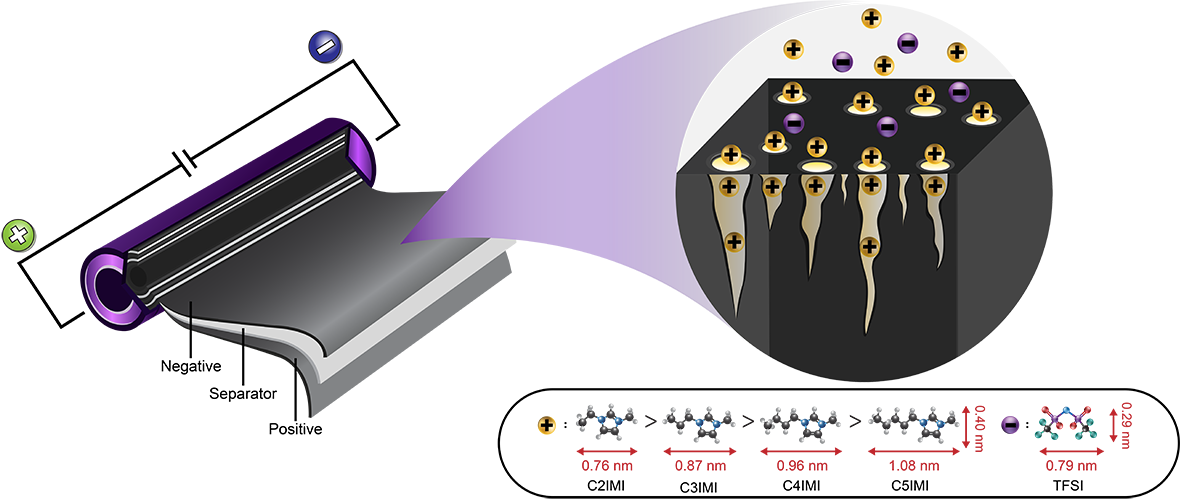

2. A Baseline Kinetic Study of Co-Free Layered Li1+ x (Ni0. 5Mn0. 5) 1-xO2 Positive Electrode Materials for Lithium-Ion Batteries
Nutthaphon Phattharasupakun, Marc Marcel Cormier, Yulong Liu, Chenxi Geng, Ines Hamam, Aaron Liu, Michel Johnson, Montree Sawangphruk, Jeff R Dahn
Journal of the Electrochemical Society, 2021, 168, 110502
DOI: https://iopscience.iop.org/article/10.1149/1945-7111/ac3157?fbclid=IwAR3for5Ozo4ofoLxw8ro5DTrdbyYwiFJUbyoiIHnNrnvyOcZWhH4OlMLD6g
Abstract: Variations of Li chemical diffusion coefficient (D_c) with voltage in a series of Co-free Li1+x(Ni0.5Mn0.5)1−xO2, 0 ≤ x ≤ 0.12, materials were systematically investigated using the recently developed "Atlung Method for Intercalant Diffusion". The effects of primary and secondary particle sizes, excess Li content, heating temperature, and synthesis atmospheres on D_c were measured. Li-ion kinetics can be enhanced by an order of magnitude by lowering the amount of Ni atoms in the Li layers (cation mixing) from 10% to 4%. Decreasing cation mixing can be accomplished by either increasing the excess Li content or heating temperature. When cycled to 4.6 V, higher specific capacities were obtained, but with a penalty to D_c due to transition metal migration to the Li layers. The primary particles control the Li diffusion length in these materials, regardless of the secondary particle size indicating that grain boundary diffusion must be very rapid. The general trends observed in this work are of great value for the development of higher Mn-containing, Co-free materials. It should be possible to increase energy/power density by making large secondary particles, composed of small primary particles to minimize the solid-state diffusion length while maximizing grain boundary diffusion.
3. Voltage-Dependent Li Kinetics Leads to Charge-Discharge Asymmetry in Co-Free Li-Rich Li1. 12Ni0. 44Mn0. 44O2 under Conditions without Transition Metal Migration
Nutthaphon Phattharasupakun, Marc Marcel Cormier, Chenxi Geng, Montree Sawangphruk, Jeff R Dahn
Journal of the Electrochemical Society, 2021, 168, 090564
DOI: https://iopscience.iop.org/article/10.1149/1945-7111/ac285e/meta
Abstract: Understanding factors that influence Li-ion kinetics in positive electrode materials is important for development of Li-ion batteries capable of operating at high rates and low temperatures. Herein, the impact of rate and operating temperature on Li diffusion was studied to explain charge-discharge asymmetry where lithiation is kinetically hindered compared to delithiation. Co-free, Li-rich Li1.12Ni0.44Mn0.44O2 materials with different secondary particle sizes of 4 and 16 µm, but nearly identical primary particle sizes, lattice parameters, and percentage of Ni atoms in the Li layers, were chosen to demonstrate how worsened Li kinetics during lithiation leads to charge-discharge asymmetry. Cells were cycled between 4.4-1.5 V to eliminate transition metal migration and to understand Li kinetics as a function of state of charge. Measurements were done on an Ultra-high precision charger to measure apparent capacity loss due to kinetic limitations. At low and moderate rates, the cell capacity was fully recovered by allowing the cell voltage to pass below 2 V where a voltage plateau corresponding to a Li2MO2-like phase was observed. Li ion diffusion measurements confirmed that Li diffusion is slower at low voltage. Taken together with the rate and temperature dependence, the charge-discharge asymmetry is accounted for solely from Li-ion kinetic limitations
4. Correlating Cation Mixing with Li Kinetics: Electrochemical and Li Diffusion Measurements on Li-Deficient LiNiO2 and Li-Excess LiNi0. 5Mn0. 5O2
Marc Marcel Cormier, Nutthaphon Phattharasupakun, Aaron Liu, Chenxi Geng, Yulong Liu, Hongyang Li, Montree Sawangphruk, Jeff R Dahn
Journal of the Electrochemical Society, 2021, 168, 090535
DOI: https://iopscience.iop.org/article/10.1149/1945-7111/ac24ba/meta
Abstract: Cation mixing in Li-based layered positive electrode materials has been reported to negatively affect the electrochemical performance and transport properties of intercalated Li. However, no previous reports have systematically correlated the impact of cation mixing (Ni atoms in the Li layer) on the electrochemical properties and Li transport. Herein, a series of Li-deficient LNO (Li1−xNi1+xO2) materials with different amounts of Ni in the Li layers ranging from ca. 1.5%–6.0% was intentionally prepared by varying the Li/Ni ratio during synthesis. An order of magnitude decrease in the Li chemical diffusion coefficient was found between samples with 1.5% and 6% Ni in the Li layer. A similar dependence of the diffusion constant on the amount of Ni in the Li layer was also observed in the Li-excess materials for x = 0, 0.04, 0.08, 0.12, suggesting that, in general, larger amounts of Ni in the Li layer will lead to worse kinetics. This work quantitatively demonstrates that the amount of Ni in the Li layer needs to be carefully considered for the development of high-energy Ni-containing layered positive electrode materials as it directly affects overall electrochemical performance, phase transitions, and Li diffusion, leading to worse kinetics and seriously hindering rate capability.
5. Revealing the impacts of oxygen defects on Zn2+ storage performance in V2O5
Jin Cao, Dongdong Zhang, Yilei Yue, Teerachote Pakornchote, Thiti Bovornratanaraks, Montree Sawangphruk, Xinyu Zhang, Jiaqian Qin
Materials Today Energy, 2021, 21, 100824
DOI: https://pubs.acs.org/doi/10.1021/acsomega.1c01908?goto=supporting-info
Abstract: Vanadium pentoxide (V2O5) featured with open-framework structure and various oxidation states is regarded as the most promising cathode of aqueous zinc-ion batteries (ZIBs), whereas sluggish Zn2+ diffusion kinetics and poor structural stability plague its further application. Herein, the oxygen defects have been introduced into V2O5 (V2O5-Od), the experimental studies and first-principles calculations reveal the oxygen defects can enlarge the interlayer spacing (7.58 Å) and significantly lower the Zn2+ diffusion energy barrier (0.72 eV), leading to favorable Zn2+ migration path and fast reactive kinetics. Moreover, a narrower bandgap (0.45 eV) and lower charge transfer resistance are obtained in V2O5-Od, thus accelerating the electron transportation and improving the Zn2+ storage performance (427.3 mAh/g at 0.1 A/g). In addition, the internal structure of V2O5 is well-maintained owing to the greatly reduced formation energy of V2O5-Od (55.04 eV), contributing to outstanding cycling stability (92.1% after 5,000 cycles at 20 A/g), outperforming numerous reported cathodes. Moreover, the pouch cell with V2O5-Od delivers admirable electrochemical performance and modular integration capabilities, suggestive of its excellent practical viability. Therefore, this research highlights the great potential of oxygen defects in designing advanced electrodes and offers a guideline for exploring the working mechanism of defective electrode materials.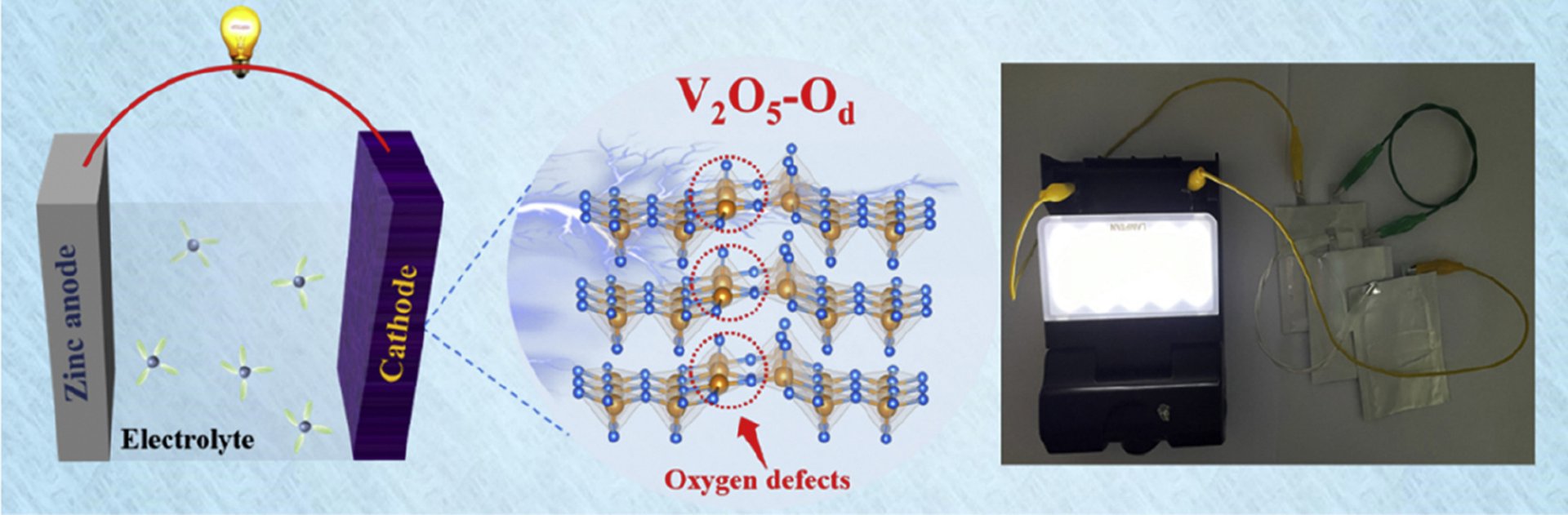

6. Scalable 18650 aqueous-based supercapacitors using hydrophobicity concept of anti-corrosion graphite passivation layer
Praeploy Chomkhuntod, Pawin Iamprasertkun, Poramane Chiochan, Phansiri Suktha, Montree Sawangphruk
Scientific Reports, 2021, 11, 13082
DOI: https://www.nature.com/articles/s41598-021-92597-y
Abstract: Scalable aqueous-based supercapacitors are ideal as future energy storage technologies due to their great safety, low cost, and environmental friendliness. However, the corrosion of metal current collectors e.g., aluminium (Al) foil in aqueous solutions limits their practical applications. In this work, we demonstrate a low-cost, scalable, and simple method to prepare an anti-corrosion current collector using a concept of hydrophobicity by coating the hydrophobic graphite passivation layer on the Al foil via a roll-to-roll coating technology at the semi-automation scale of production pilot plant of 18,650 cylindrical supercapacitor cells. All qualities of materials, electrodes, and production process are therefore in the quality control as the same level of commercial supercapacitors. In addition, the effects of the graphite coating layer have been fundamentally evaluated. We have found that the graphite-coated layer can improve the interfacial contact without air void space between the activated carbon active material layer and the Al foil current collector. Importantly, it can suppress the corrosion and the formation of resistive oxide film resulting in better rate capability and excellent cycling stability without capacitance loss after long cycling. The scalable supercapacitor prototypes here in this work may pave the way to practical 18,650 supercapacitors for sustainable energy storage systems in the future.
7. First-Principle study of lithium polysulfide adsorption on heteroatom doped graphitic carbon nitride for Lithium-Sulfur batteries
Nattida Yamsang, Jarinya Sittiwong, Pemika Srifa, Bundet Boekfa, Montree Sawangphruk, Thana Maihom, Jumras Limtrakul
Applied Surface Science, 2021, 565, 150378
DOI: https://doi.org/10.1016/j.apsusc.2021.150378
Abstract: The intermediate lithium polysulfide species (LiPSs) suppressing dissolution into liquid electrolyte has been one of the important issues for large-scale commercial applications of Lithium-sulfur batteries (LSBs). Here, we perform periodic density functional theory calculations to reveal the potential of graphitic carbon nitride nanosheet (g-C3N4) and its heteroatoms (B, O, P and S) doped systems, to be used as chemical anchoring materials for LiPSs. From the calculations, the LiPSs are stabilized on g-C3N4 materials via lithium bond interaction between Li of LiPSs and N of g-C3N4. With the introduction of dopant atoms, tuning possibility of the LiPSs adsorption energies is observed. The interaction of LiPSs on B- and P-doped g-C3N4 provide optimum adsorption energies which is appropriate for LSBs anchoring. However, the O- and S-doped g-C3N4 have a weak adsorption which is similar to the pristine one. The anchoring performance of g-C3N4 materials for suppressing LiPSs in dissolving into the electrolytes is also highlighted by comparing their adsorption on g-C3N4 materials and on various electrolytic clusters. The adsorption energies of LiPSs on B- and P-doped g-C3N4 are found to be stronger than those on electrolytes. The electron transfers and van der Waals interaction are found to play significant roles for the LiPSs adsorption. The results provide the fundamental understanding g-C3N4 surface tuning by doping heteroatoms for the suppression materials to reduce the shuttle effect, which will be beneficial for the advanced LSBs development.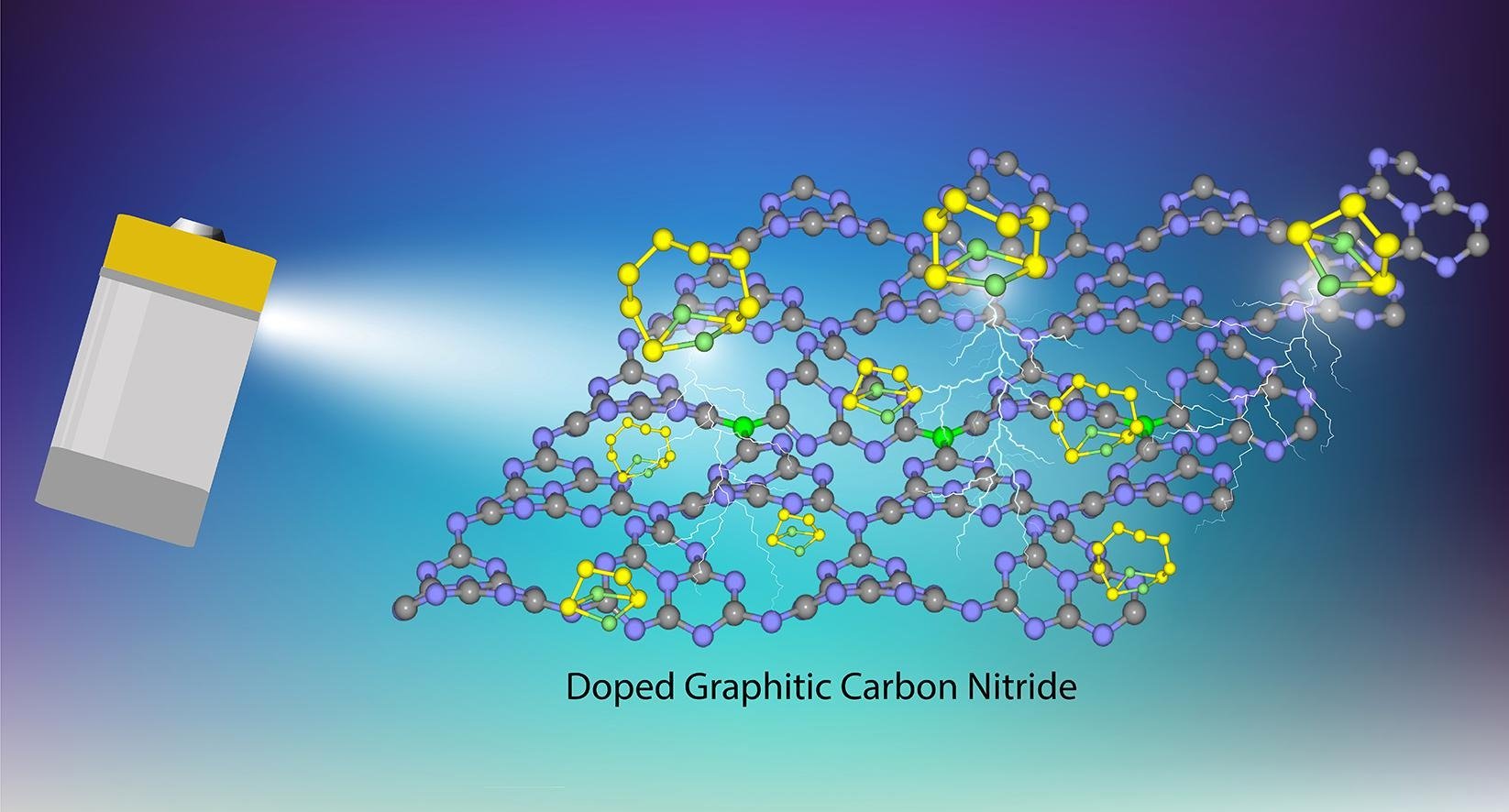

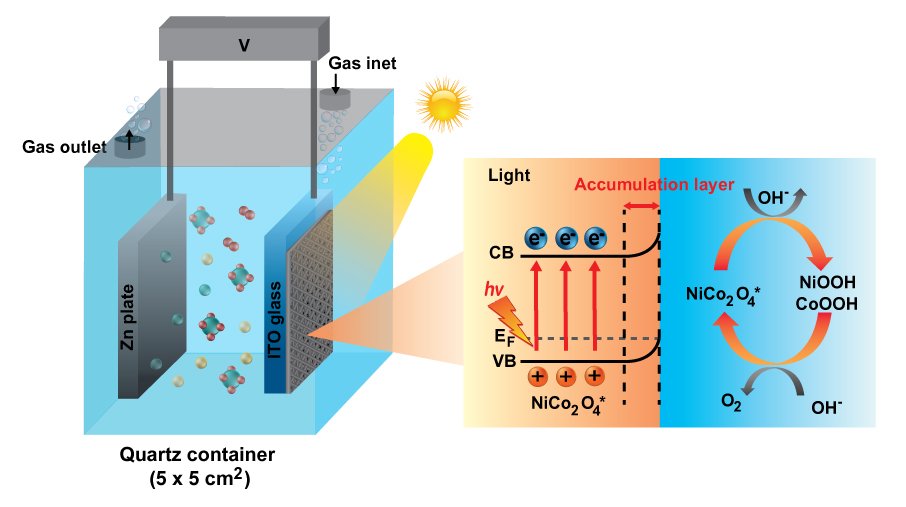
8. Insight into photoelectrocatalytic mechanisms of bifunctional cobaltite hollow-nanofibers towards oxygen evolution and oxygen reduction reactions for high-energy zinc-air batteries
Chanikarn Tomon, Sangchai Sarawutanukul, Nutthaphon Phattharasupakun, Salatan Duangdangchote, Praeploy Chomkhundtod, Pinit Kidkhunthod, Montree Sawangphruk
Electrochimica Acta, 2021, 392, 139022
DOI: https://doi.org/10.1016/j.electacta.2021.139022
Abstract: Using the photoelectrocatalyst to facilitate the oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) for the charge storage enhancement of Zn-air batteries (ZABs) has not yet been widely investigated. This work presents bimetal cobaltite hollow nanofiber electrocatalysts as the photocathodes in the ZABs. Among all catalysts, the NiCo2O4 hollow nanofiber exhibits superior performances towards OER and ORR. Under light irradiation (AM1.5), the NiCo2O4 can absorb photon energy and generate free electrons and photogenerated carriers or holes, reducing the energy barrier enhancing the kinetic activity of OER/ORR. The NiCo2O4 hollow nanofiber decreases the OER and ORR overpotentials for 14.3% and 8.1%, respectively and improves the OER and ORR kinetics for 19.3% and 20.8%, respectively compared with those under dark condition. The in situ electrochemical X-ray absorption spectroscopy results reveal that light can enhance both Ni and Co catalytic activities but Ni is found to be the main active species for the OER. The as-fabricated ZAB cell with the NiCo2O4 photocathode exhibits the decreasing voltage gap from 0.85 V (Dark) to 0.64 V (Light) due to the highly active hole and the excited electron increasing the specific capacity up to 11.4% as compared to that under dark condition.

9. Reducing the Energy Band Gap of Cobalt Hydroxide Nanosheets with Silver Atoms and Enhancing Their Electrical Conductivity with Silver Nanoparticles
Montakan Suksomboon, Ketsuda Kongsawatvoragul, Salatan Duangdangchote, and Montree Sawangphruk
ACS Omega, 2021, 6, 20804-20811
DOI: https://pubs.acs.org/doi/10.1021/acsomega.1c01908
Abstract: Although cobalt hydroxide (Co(OH)2) has been attracting attention in several applications, its photoelectrochemical property has not yet been fully investigated. In this work, tuning the energy band gap of Co(OH)2 nanosheets with silver atoms and enhancing their electrical conductivity with silver nanoparticles were then focused. A Ag-doped α-Co(OH)2 thin film was successfully synthesized via an electrodeposition method. The optical properties of the as-prepared materials were characterized by UV–vis and fluorescence lifetime spectroscopies and further confirmed by density functional theoretical calculation. It was found that Ag atoms between adjacent layers of Co(OH)2 can reduce its electronic band gap to 2.45 eV (α-Co(OH)2) as compared to 2.85 eV of β-Co(OH)2. In terms of electrochemical properties, silver nanoparticles (AgNPs) can enhance the electrical conductivity of Co(OH)2 nanosheets, leading to faster charge transfer reducing the internal resistance and significantly increasing the overall charge storage performance. Interestingly, under light illumination, Ag-doped α-Co(OH)2 exhibits ca. 0.8 times lower charge storage capacity as compared to that under the dark condition. This is because the photoelectrons can be recombined with the generated holes in the conduction band. The charge storage mechanisms of Ag-doped α-Co(OH)2 operated under dark conditions and light irradiation were further studied and confirmed using in situ electrochemical X-ray absorption spectroscopy (XAS). Overall, the in situ XAS supports the electrochemical result. This finding may pave a way to further develop photoactive advanced functional materials of metal hydroxides and oxides.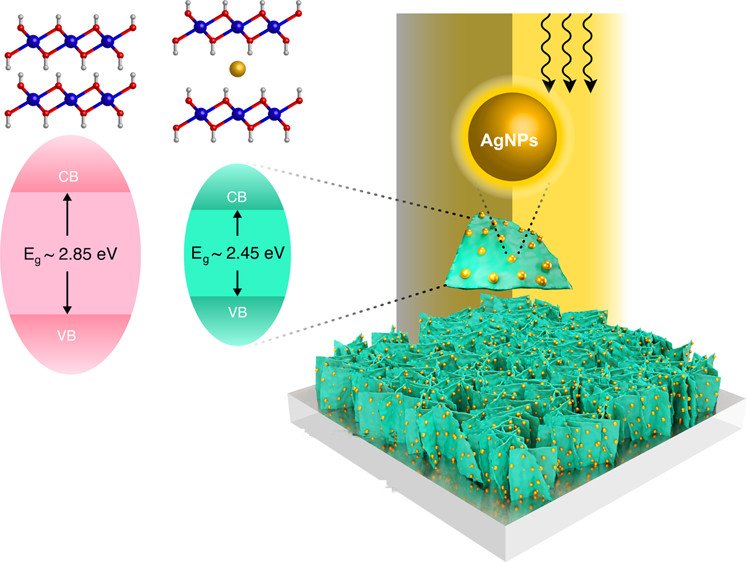

10. Factors that Affect Capacity in the Low Voltage Kinetic Hindrance Region of Ni-Rich Positive Electrode Materials and Diffusion Measurements from a Reinvented Approach
Aaron Liu6, Nutthaphon Phattharasupakun6, Marc M. E. Cormier, Eniko Zsoldos, Ning Zhang, Erin Lyle1, Phillip Arab, Montree Sawangphruk and J. R. Dahn
Journal of the Electrochemical Society,2021, 168, 070503
DIO: https://iopscience.iop.org/article/10.1149/1945-7111/ac0d69
Abstract: With research continuing to push for higher Ni content in positive electrode materials, issues such as the 1st cycle irreversible capacity and kinetic hindrances related to Li diffusion become more significant. This work highlights the impact of various material parameters on electrochemical performances, specifically the kinetic hindrances to Li diffusion in the low voltage region. Increasing the amount of substituents, increasing the secondary particle size and increasing the primary particle size were all variables found to decrease capacity in the ∼3.4–3.6 V region at modest discharge rates and increase the 1st cycle IRC. The capacity in the ∼3.4–3.6 V region can be recovered when cycling at a higher temperature at similar discharge rates or when cycling to a low cut-off voltage of 2 V. Since these processes are related to the diffusion of Li in the positive electrode, analysis of the Li chemical diffusion coefficient, Dc, is presented using a reinvented approach we call the "Atlung Method for Intercalant Diffusion." The measured Dc for the single crystalline LiNi0.975Mg0.025O2 materials were found to be about 2 orders of magnitude smaller compared to the polycrystalline materials if the secondary particle size was used in the calculation of Dc for the polycrystalline samples. If the primary particle size of the polycrystalline materials was used, then Dc was similar to the single crystal materials. These results demonstrate that lattice diffusion is much slower compared to grain boundary diffusion offering insight for optimizing material morphology for better rate performance.
11. Optimization of the Electrode Properties for High-performance Ni-rich Li-ion Batteries
Sangchai Sarawutanukul, Chanikarn Tomon, Nutthaphon Phattharasupakun, Salatan Duangdangchote, Farkfun Duriyasart, Poramane Chiochan, Montree Sawangphruk
ACS Applied Materials & Interfaces, 2021, 13, 30643–30652
DOI: https://pubs.acs.org/doi/10.1021/acsami.1c07019
Abstract: The microstructure of the electrodes in lithium-ion batteries (LIBs) strongly affects their gravimetric and volumetric energy and power as well as their cycle life. Especially, the effect of the microstructure in the case of next-generation Ni-rich cathode materials has not yet been investigated. A comprehensive understanding of the calendering process is therefore necessary to find an optimal level of the electrode microstructure that can enhance lithium-ion transportation, minimize plastic deformation, and improve conductivity. This work therefore aims to investigate the effect of microstructure and wettability on the electrode kinetics of next-generation Ni-rich LiNi0.88Co0.09Al0.03O2-based 18650 cylindrical cells, which were produced at the semiautomation scale of the pilot plant. Thus, all materials, electrodes, and the battery production are in quality control as the same level of commercial LIBs. With the optimized microstructure and other properties including a finely tuned compaction degree of 17.54%, a thickness of 188 μm, a sheet resistivity of 36.47 mΩ cm–2, a crystallite size of 88.85 nm, a porosity of 26.03%, an electrode Brunauer–Emmett–Teller (BET) surface area of 1.090 m2 g–1, an electrode density of 2.529 g cm–3, and an electrolyte uptake capability of 47.8%, the optimized LiNi0.88Co0.09Al0.03O2 18650 cylindrical cells exhibit excellent high-rate capacity retention, fast Li-ion diffusion, and low internal resistance. The optimized electrode microstructure of next-generation Ni-rich cathode materials could be an effective strategy toward the real application of next-generation Ni-rich LIBs.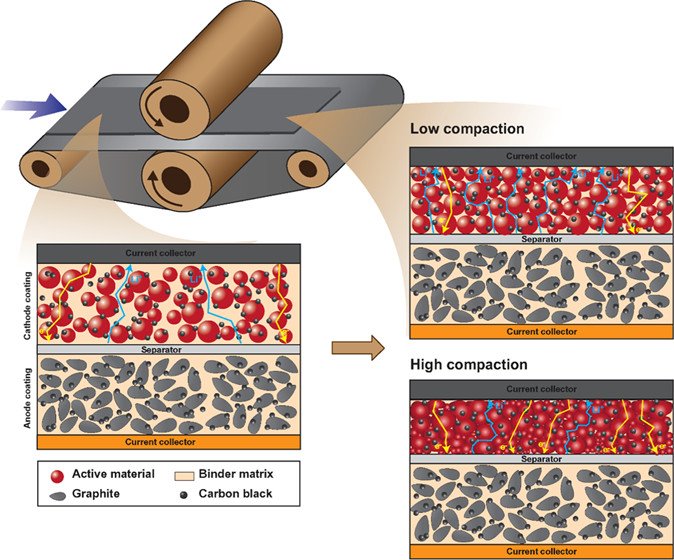

12. Effect of fluoroethylene carbonate on transport property of the electrolyte towards Ni-rich Li-ion batteries with high safety
Salatan Duangdangchote, Nutthaphon Phattharasupakun, Praeploy Chomkhuntod, Poramane Chiochan, Sangchai Sarawutanukul, Chanikarn Tomon, Nattanon Joraleechanchai, Montree Sawangphruk
Chemical Communications, 2021, 57, 6732-6735
DOI: https://doi.org/10.1039/D1CC02120F
Abstract: Transport phenomena and the solvation structure of lithium ions (Li+) and hexafluorophosphate anions (PF6−) in the electrolyte with different fluoroethylene carbonate (FEC) concentrations as well as the electrochemical performance and safety of the Ni-rich Li-ion battery cells at the 18650 cylindrical cell level are investigated. We have found that the electrolyte with an optimized FEC concentration (25 %v/v) can effectively enhance the transport property in terms of Li+ transference number and contact ion pair (CIP) ratio leading to high performance and safety of practical 18650 cylindrical LIBs.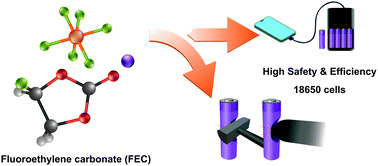

13. Solar-driven energy storage enhancement of nickel hydroxide nanomaterials
Ketsuda Kongsawatvoragul, Praeploy Chomkhuntod, Montree Sawangphruk
Electrochimica Acta, 2021, 388, 138654
DOI: https://doi.org/10.1016/j.electacta.2021.138654
Abstract: Nickel hydroxide (Ni(OH)2) is one of the most promising cathode materials that are widely used in rechargeable batteries, for instance, the nickel-metal hydride battery (NiMH). The challenge relating to Ni(OH)2 is the charge transfer process during the electrochemical reaction. In this work, Ni(OH)2 was explored as both photo-harvesting and energy storage materials under UV-visible light (AM1.5) illumination to enhance the charge transfer process for the electrochemical reaction of Ni(OH)2 via the photovoltaic effect. The generation of polaron, an electron-hole pair of Ni(OH)2, under light and potential bias increases the charge carrier density for the electrochemical reaction. The result suggests that the capacity was enhanced by 14.4% compared with that under the dark condition. This new finding may further improve the charge storage capacity and development of NiMH and others using metal oxide and hydroxide materials as electrode materials for sustainability.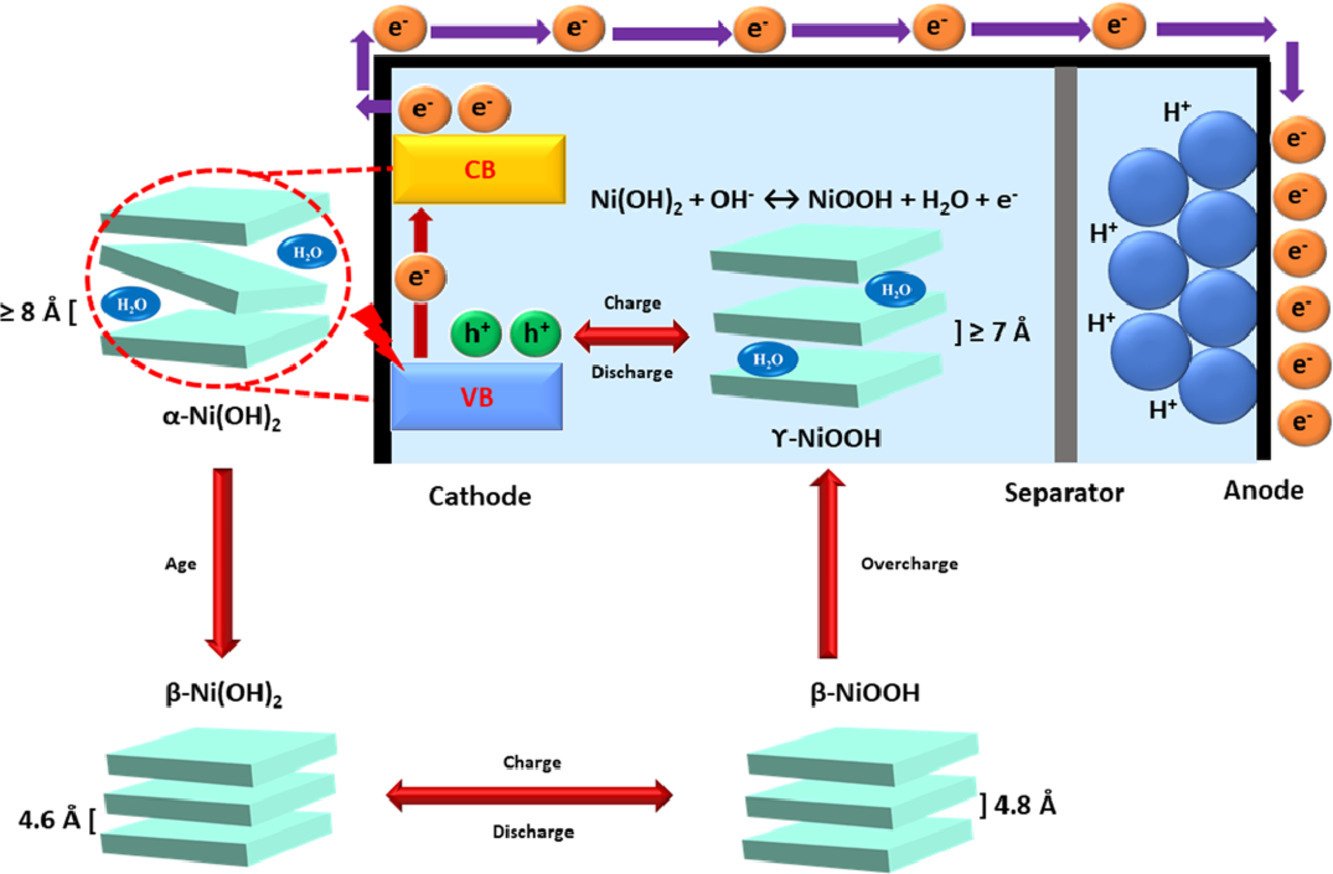

14. The electrochemistry of size dependent graphene via liquid phase exfoliation: capacitance and ionic transport
Varisara Deerattrakul, Wisit Hirunpinyopas, Nuttapon Pisitpipathsin, Thanit Saisopa, Montree Sawangphruk, Chakrit Nualchimplee, Pawin Iamprasertkun
Physical Chemistry Chemical Physics, 2021, 23, 11616-11623
DOI: https://doi.org/10.1039/D1CP00887K
Abstract: Recently, graphene-based materials have become ubiquitous in electrochemical devices including electrochemical sensors, electrocatalysts, capacitive and membrane desalination and energy storage devices. However, many of the electrochemical properties of graphene (particularly the capacitance and ionic transport) are not yet fully understood. This paper explores the capacitance and ionic transport properties of size dependent graphene (from 100 nm to 1 μm) prepared through the liquid phase exfoliation of graphite in which the size of graphene was finely selected using a multi-step centrifugation technique. Our experiment was then expanded to include basal plane graphene using highly ordered pyrolytic graphite as a model electrode, describing the assumed theoretical graphene capacitance (quoted as 550 F g−1 or 21 μF cm−2) and the electrochemical surface area of the carbon-based materials. This work improves our understanding of graphene electrochemistry (capacitance and ion transport), which should lead to the continuing development of many high-performance electrochemical devices, especially supercapacitors, capacitive desalination and ion-based selective membranes.

15. Insight into the effect of ionic liquid-based additives at the solid electrolyte interphase for lithium metal batteries
Nattanon Joraleechanchai, Salatan Duangdangchote, Montree Sawangphruk
Journal of the Electrochemical Society, 2021, 168, 040534
DOI: https://iopscience.iop.org/article/10.1149/1945-7111/abf7e3/meta
Abstract: We have investigated the effect of ionic liquids (ILs) additives in 1M LiTFSI in 1,3-dioxolane (DOL)/dimethyl ether (DME) (1:1, v/v) on the solid electrolyte interphase (SEI) using a combined theoretical and experimental method for next-generation Li metal batteries. Among all investigated cations of ILs including imidazolium (IMI+), pyrrolidinium (PYR+), and piperidinium (PIP+) cations, we have observed that the presence of IMI+ provides relatively larger decomposed products of the TFSI anion on the Li metal surface when compared with saturated-ring type cation ILs including PYR+- and PIP+-ILs due to its high electron affinity (EA). Furthermore, the reactive molecular dynamics results show that the SEI layer of the system using the ILs with saturated rings is significantly thin when compared to that of the ILs with aromatic-ring, which is desired for fast Li-ion diffusivity through the SEI. This finding may lead to an ideal design of electrolyte system for future Li-metal batteries.
16. Enhancing bifunctional electrocatalysts of hollow Co3O4 nanorods with oxygen vacancies towards ORR and OER for Li–O2 batteries
Chanikarn Tomon, Atiweena Krittayavathananon, Sangchai Sarawutanukul, Salatan Duangdangchote, Nutthaphon Phattharasupakun, Kan Homlamai, Montree Sawangphruk
Electrochimica Acta, 2021, 367, 137490
DOI: https://doi.org/10.1016/j.electacta.2020.137490
Abstract: The Co3O4, which is on the top of volcano plot, having high oxygen vacancy of ca. 30% finely tuned promotes narrow band gap, resulting in the facilitation of the electron and charge transportation. The as-synthesized Co3O4 catalyst can improve the electrocatalytic activity towards oxygen evolution reaction (OER) and oxygen reduction reaction (ORR). The as-prepared catalyst exhibits the superior ORR/OER stability for which the relative current decays only 7% and 14% for OER and ORR, respectively. By contrast, the OER stability of the RuO2 catalyst presents the significant decay in relative current about 20%. The ORR stability of Pt/C also remarkably decreases to 29%. The catalyst here can be used as an efficient bifunctional catalyst at the cathode of Li–O2 battery. It provides the superior performance as compared to that using the state-of-the-art Pt/C and RuO2/C electrocatalysts.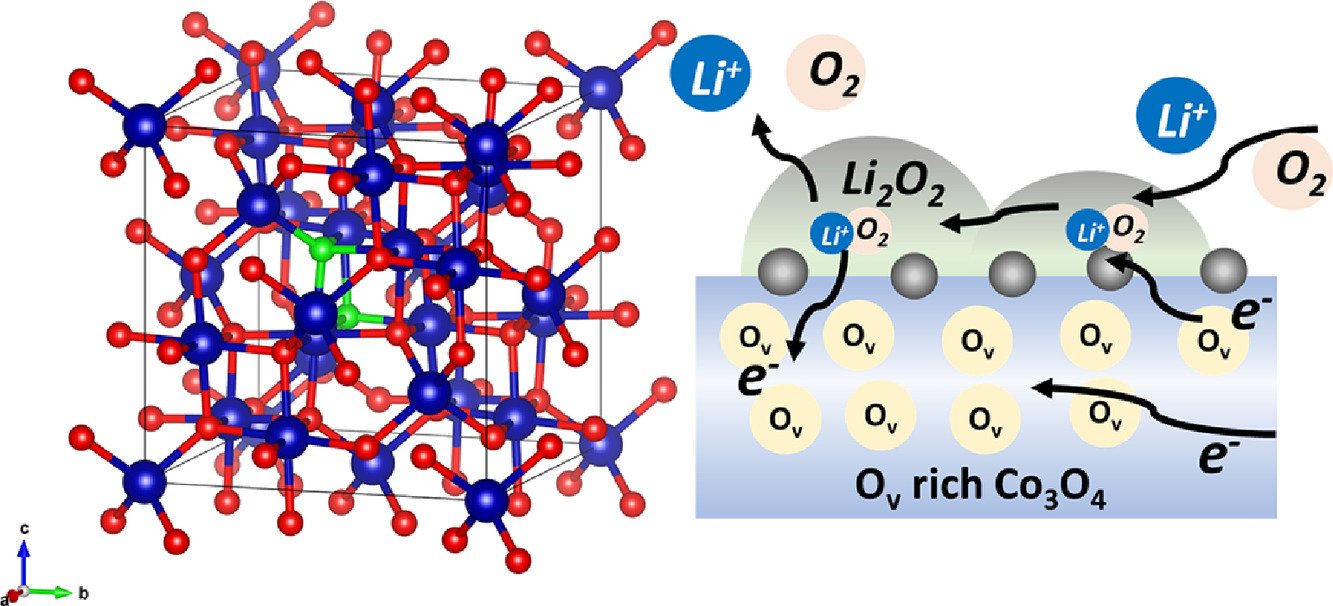

17. Controlling the flake size of bifunctional 2D WSe2nanosheets as flexible binders and supercapacitor materials
Pawin Iamprasertkun, Wisit Hirunpinyopas, Varisara Deerattrakul, Montree Sawangphruk, Chakrit Nualchimplee
Nanoscale Advances, 2021, 3, 653-660
DOI: https://doi.org/10.1039/D0NA00592D
Abstract: A new approach using graphene as a conductive binder in electrical supercapacitors has recently been proposed. Graphene shows outstanding properties as a conductive binder, and can be used to replace conductive, additive, and polymer binders. However, graphene follows an EDLC behaviour, which may limit its electrochemical performance. In the process described in this work, we introduced WSe2 nanoflakes as a new approach to using pseudocapacitive materials as binders. The WSe2 nanoflakes were produced through liquid phase exfoliation of bulk WSe2, and the flake size was finely selected using a controlled centrifugation speed. The physical and electrochemical properties of the exfoliated WSe2 flakes were analysed; it was found that the smallest flakes (an average flake size of 106 nm) showed outstanding electrochemical properties, expanding our understanding of transition metal dichalcogenide (TMD) materials, and we were able to demonstrate the applicability of using WSe2 as a binder in supercapacitor electrodes. We also successfully replaced conductive additives and polymer binders with WSe2. The overall performance was improved: capacitance was enhanced by 35%, charge transfer resistance reduced by 73%, and self-discharge potential improved by 9%. This study provides an alternative application of using TMD materials as pseudo capacitive binders, which should lead to the continued development of energy storage technology.
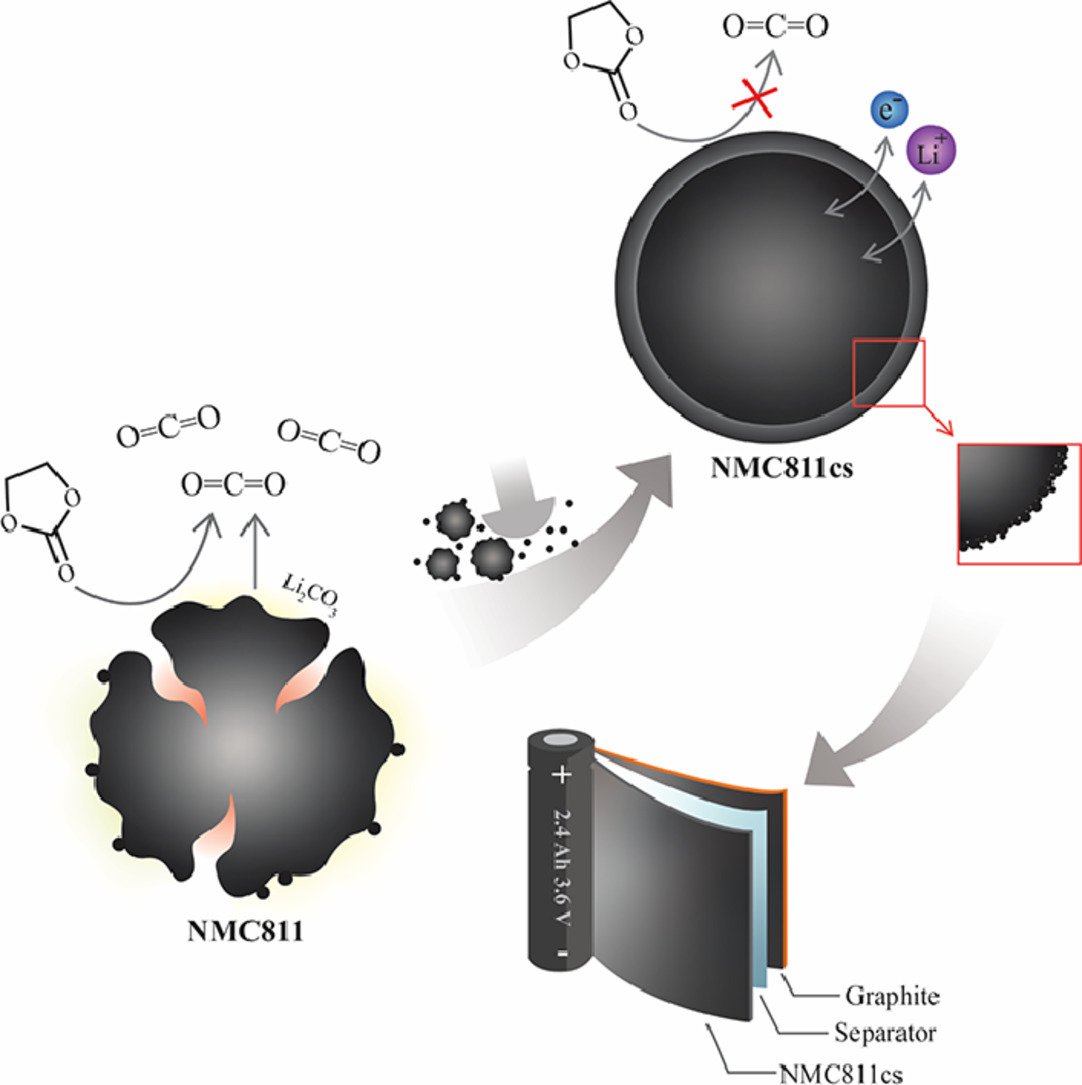
18. Core-shell Ni-rich NMC-Nanocarbon cathode from scalable solvent-free mechanofusion for high-performance 18650 Li-ion batteries
Nutthaphon Phattharasupakun, Juthaporn Wutthiprom, Salatan Duangdangchote, Sangchai Sarawutanukul, Chanikarn Tomon, Farkfun Duriyasart, Suchakree Tubtimkuna, Chalita Aphirakaramwong, Montree Sawangphruk
Energy Storage Materials, 2021, 36, 485-495
DOI: https://doi.org/10.1016/j.ensm.2021.01.032
Abstract: Although the Ni-rich LiNi0.8Mn0.1Co0.1O2 (NMC811) cathode can store high electrical energy suitable for powering long-range electric vehicles, its poor cycling stability still hinders the practical application. Herein, a surface coating approach using a green scalable solvent-free mechanofusion process is introduced to prepare an infusing thin layer carbon nanosphere shell to improve the intrinsic drawbacks of NMC811 including poor electrical conductivity, Li+/Ni2+ cation mixing, parasitic reactions with electrolytes, and micro-crack formation. The in operando XRD of NMC811@carbon core@shell so-called NMC811cs reveals the smooth and fast phase transition through H1-H2-H3 with higher Li utilization and better stability even though it shows larger abrupt change in c lattice parameter compared to the pristine NMC811. In addition, the onset potential of CO2 and H2 evolution is found to be extended by the carbon coating as observed by an online Differential Electrochemical Mass Spectrometry. Finally, the practical application of the NMC811cs is demonstrated by the 18650 battery prototype which shows higher specific capacity and energy as well as excellent cycling stability compared to the pristine NMC811. This work provides both fundamental understanding and economical strategy towards highly stable and practical high-energy Li-ion batteries.
Graphical abstract