By: Suktha, Phansiri; Chiochan, Poramane; Iamprasertkun, Pawin; et al.
Electrochimica Acta, 2015, 176, 504-513
DOI: https://www.sciencedirect.com/science/article/pii/S0013468615301122?via%3Dihub
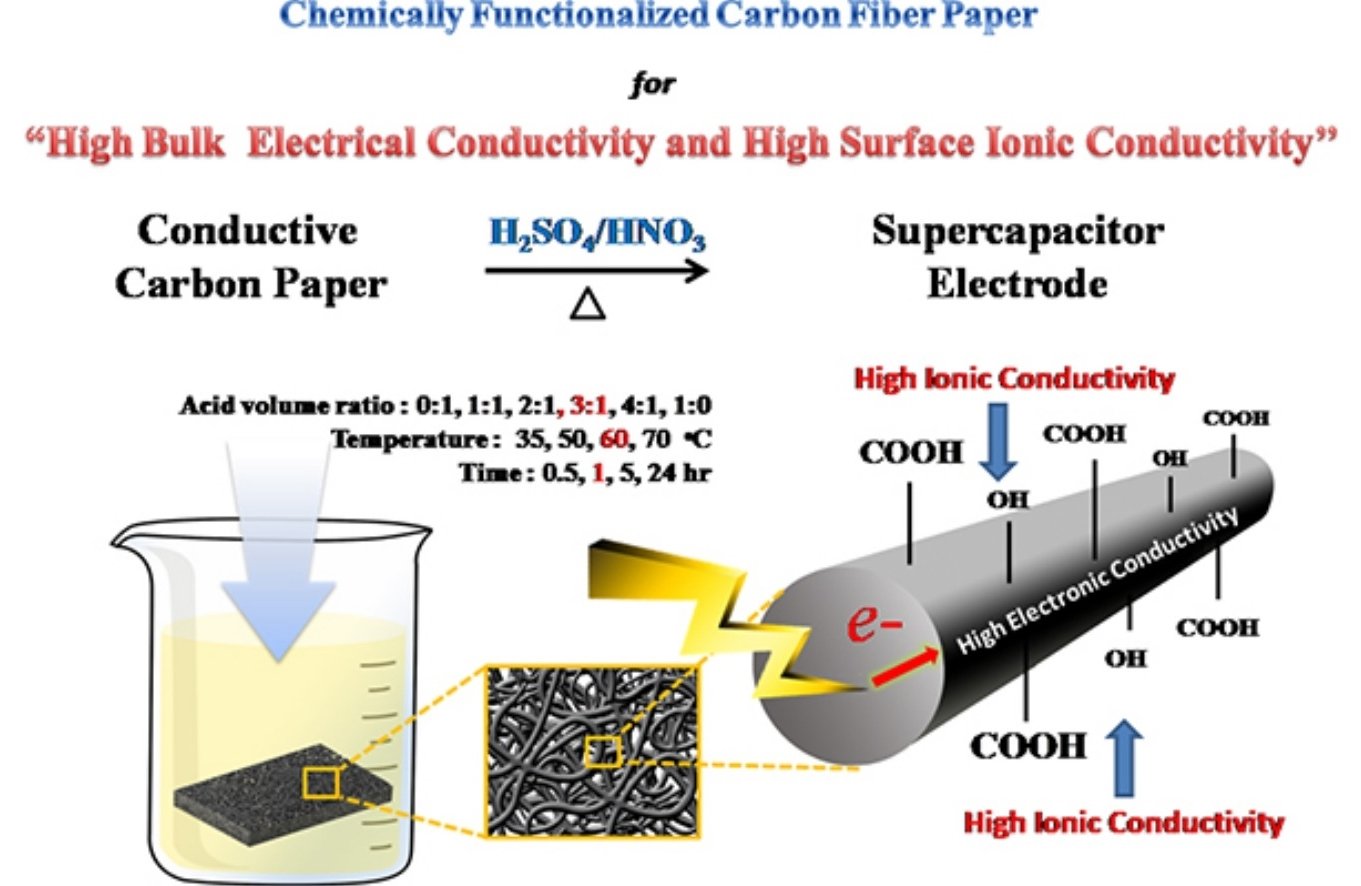
Abstract: Although carbon fiber paper (CFP) or nonwovens are widely used as a non-corrosive and conductive substrate or current collector in batteries and supercapacitors as well as a gas diffusion layer in proton exchange membrane fuel cells, the CFP cannot store charges due to its poor ionic conductivity and its hydrophobic surface. In this work, the chemically functionalized CFP (f-CFP) consisting of hydroxyl and carboxylic groups on its surface was produced by an oxidation reaction of CFP in a mixed concentrated acid solution of H2SO4:HNO3 (3:1 v/v) at 60 °C for 1 h. Other amide and amine groups modified CFP were also synthesized for comparison using a dehydration reaction of carboxylic modified CFP with ethylenediamine and n-butylamine. Interestingly, it was found that hydroxyl and carboxylic groups modified CFP behave as a pseudocapacitor electrode, which can store charges via the surface redox reaction in addition to electrochemical double layer capacitance. The aqueous-based supercapacitor of f-CFP has high areal, volumetric, and specific energy (49.0 μW.h/cm2, 1960 mW.h/L, and 5.2 W.h/Kg) and power (3.0 mW/cm2, 120 W/L, and 326.2 W/Kg) based on the total geometrical surface area and volume as well as the total weight of positive and negative electrodes. High charge capacity of the f-CFP stems from high ionic charge and pseudocapacitive behavior due to hydroxyl and carboxylic groups on its surface and high bulk electronic conductivity (2.03 mS/cm) due to 1D carbon fiber paper. The acid treatment process here could be used to improve the charge storage capacity of other carbonaceous materials such as carbon nanotubes.

2. High-performance supercapacitor of electrodeposited porous 3D polyaniline nanorods on functionalized carbon fiber paper: Effects of hydrophobic and hydrophilic surfaces of conductive carbon paper substrates
By: Kaewsongpol, Tanon; Sawangphruk, Montree; Chiochan, Poramane; et al.
MATERIALS TODAY COMMUNICATIONS, 2015, 4, 176-185
DOI : https://www.sciencedirect.com/science/article/pii/S2352492815300179?via%3Dihub
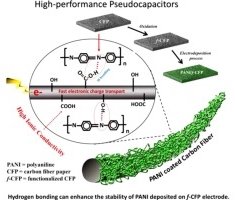
Abstract: Although polyaniline (PANI) was electrodeposited on various conductive substrates for use as the pseudocapacitor electrodes, the effect of carbon paper surfaces has not yet been investigated. In this work, PANI was electrodeposited on two carbon surfaces including hydrophobic carbon fiber paper (CFP) and hydrophilic functionalized CFP (f-CFP). f-CFP with a bulk conductivity of 2.03 mS/cm having hydroxyl and carboxyl groups on its surface can significantly enhance the charge storage performance of the PANI/f-CFP pseudocapacitors due to its high surface ionic conductivity. The symmetric pseudocapacitors of PANI/f-CFP in aqueous and organic electrolytes exhibit much higher areal capacitance, power and energy than those of PANI coated on the hydrophobic surface of CFP. The areal capacitance of a half-cell PANI/f-CFP electrode in 1 M H2SO4 (aq.) is 704 mF/cm2 (1323 F/g_PANI) at 10 mV/s. A symmetric organic-based PANI/f-CFP pseudocapacitor at an areal power of 240 μW/cm2 (a specific power of 351 W/kg_PANI) has an areal energy of 102 μWh/cm2 (a specific energy of 149 Wh/kg_PANI). At a lower areal energy of 2.0 μWh/cm2 (3 Wh/kg_PANI), the device has higher areal power of 2660 μW/cm2 (3881 W/kg_PANI). The stability of the device tested by galvanic charge–discharge over 2500 cycles is over 97% capacitance retention.

3. Solid-type supercapacitor of reduced graphene oxide-metal organic framework composite coated on carbon fiber paper
By: Srimuk, Pattarachai; Luanwuthi, Santamon; Krittayavathananon, Atiweena; et al.
Electrochimica Acta, 2015, 157, 69-77
DOI : https://www.sciencedirect.com/science/article/pii/S0013468615001024?via%3Dihub
Abstract: A composite material of reduced graphene oxide (rGO) and Hong Kong University of Science and Technology-1 (HKUST-1) metal organic framework (MOF) was produced and used as the supercapacitor electrode material. Pure HKUST-1 has a microporous structure with poor conductivity limiting its electrochemical applications while loading rGO to HKUST-1 leads to a novel composite with a mesoporous structure and good electrochemical properties. 10 wt.% rGO/HKUST-1 has a high BET surface area of 1241 m2 g−1, a specific pore volume of 0.78 cm3 g−1, and an average pore diameter of 8.2 nm, which is a proper pore size to allow uptaking and releasing of electrolytes. A half-cell electrode of 10 wt.% rGO/HKUST-1 coated on flexible carbon fiber paper (CFP) exhibits a high specific capacitance of 385 F g−1 at 1 A g−1 while pure HKUST-1 stores only 0.5 F g−1. A 1.8″ × 4.0″ symmetric solid-type supercapacitor of the 10 wt.%rGO/HKUST-1 exhibits a specific power of 3100 W kg−1 and a specific energy of 42 Wh kg−1. The supercapacitor can practically supply electricity to a spinning 3-V motor over 9-min discharging time.

4. In situ synthesis of permselective zeolitic imidazolate framework-8/graphene oxide composites: rotating disk electrode and Langmuir adsorption isotherm
By: Luanwuthi, Santamon; Krittayavathananon, Atiweena; Srimuk, Pattarachai; et al.
RSC ADVANCES, 2015, 5, 46617-46623
DOI : https://pubs.rsc.org/en/content/articlelanding/2015/RA/C5RA05950J#!divAbstract
Abstract: Although zeolitic imidazolate framework-8 (ZIF-8) has been well known and widely used in gas storage and catalysis applications, its electrochemical application has not yet been well investigated due to its poor conductivity limiting the electronic charge transfer. In this work, graphene oxide (GO) has been loaded to ZIF-8 using an in situ synthesis in order to improve its conductivity. The morphological, structural and electrochemical properties of GO/ZIF-8 composites were studied by scanning electron and transmission electron microscopies, X-ray photoelectron and absorption spectroscopies, voltammetry, rotating disk electrode, and Langmuir adsorption isotherm. It was found that a 2 wt% loading content of GO to ZIF-8 shows high permselective property to redox probe mediators enhancing the Faradaic current of ferrocene methanol (FcOH) but reducing the Faradaic current of Fe(CN)64−. Gibbs's free energy (ΔG0) of the adsorption of FcOH within the 2 wt%GO/ZIF-8 film is −16.81 kJ mol−1 indicating the spontaneous adsorption process. This composite may lead to many useful electrochemical applications of ZIF-8 especially in a research area of the permselective membrane.