1. Metalloporphyrin-Based Metal–Organic Frameworks on Flexible Carbon Paper for Electrocatalytic Nitrite Oxidation
Pila, T., Chirawatkul, P., Piyakeeratikul, P., Somjit, V., Sawangphruk, M., Kongpatpanich, K.
Chemistry - A European Journal, 2020, 26, 17399–17404
DOI: https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/chem.202003206
Abstract: Deposition of redox-active metal–organic frameworks (MOFs) as thin films on conductive substrates is of great importance to improve their electrochemical performance and durability. In this work, a series of metalloporphyrinic MOF crystals was successfully deposited as thin films on carbon fiber paper (CFP) substrates, which is an alternative to rigid glass substrates. The specific dimensions of the obtained films could be adjusted easily by simple cutting. Metalloporphyrinic MOFs on CFP with different active metal species have been employed for electrochemical conversion of the carcinogenic nitrite into the less toxic nitrate. The MOFs on CFP exhibit remarkable improvement in terms of the electrocatalytic performance and reusability compared with the electrodes prepared from MOF powder. The contribution from metal species of the porphyrin units and reaction mechanisms was elucidated based on the findings from X-ray photoelectron spectroscopy (XPS) and in situ X-ray absorption near edge structure (XANES) measured during the electrochemical reaction. By integrating the redox-active property of metalloporphyrinic MOFs and high conductivity of CFP, MOF thin films on CFP provided a significant improvement of electrocatalytic performance to detoxify the carcinogenic nitrite with good stability.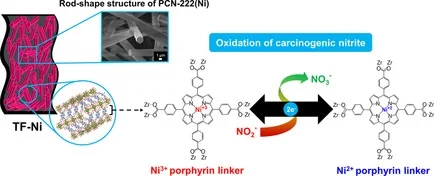

2. Impact of Cr doping on the voltage fade of Li-Rich Mn-Rich Li1.11Ni0.33Mn0.56O2 and Li1.2Ni0.2Mn0.6O2 positive electrode materials.
Phattharasupakun, N., Geng, C., Johnson, M.B., Väli, R., Sawangphruk, M., Dahn, J.R.
Journal of The Electrochemical Society, 2020, 167, 160545
DOI: https://iopscience.iop.org/article/10.1149/1945-7111/abd44e
Abstract: Voltage fade during charge-discharge cycling in Layered Li-rich Mn-rich positive electrode materials needs to be overcome for the development of high-energy low cost Li-ion batteries. Several cation dopants have been introduced into the bulk lattice to mitigate voltage decay by limiting transition metal (TM) migration, inhibiting phase transformation, or reducing the extent of oxygen release. Here, a series of electrochemically active Cr substituted (2.5, 5.0, and 10 mol%) Co-free Li1.11Ni0.33Mn0.56O2 and Li1.2Ni0.2Mn0.6O2 compositions were synthesized via dry particle fusion followed by heat treatment with Li2CO3. Cr doping improves specific capacity and capacity retention via multiple electron transfer of Cr3+/Cr6+ as well as mitigates voltage fading to a certain extent. The impact of Cr on voltage decay was studied by careful measurements of dQ/dV vs V on Cr-doped and undoped samples before and after cycle testing.
3. Graphene Aerogels with Ultrahigh Pore Volume for Organic Dye Adsorption and High-Energy Lithium Batteries
Sroysee, W., Suktha, P., Kongsawatvoragul, K., Vadivel, S., Sawangphruk, M.
Industrial and Engineering Chemistry Research, 2020, 59, 20719-20729
DOI: https://pubs.acs.org/doi/10.1021/acs.iecr.0c04026
Abstract: Three-dimensional (3D) hierarchical reduced graphene oxide (rGO) aerogel with a finely tuned C/O ratio of 11.8 can be used for various applications because of its high specific surface area (925.27 m2 g-1), specific pore volume of 6.46 cm3 g-1, and light weight as well as excellent electrical conductivity and electrochemical property. The permselectivity of the 3D rGO aerogels with different C/O ratios was investigated using neutral, positive, and negative redox mediators. The neutral and cationic redox mediators permselectively adsorb within the rGO aerogels via π-πand noncovalent electrostatic interactions, respectively. In contrast, the anionic redox mediator does not adsorb within the 3D rGO aerogels due to the repulsion force. The 3D rGO can be used as an absorbent for toxic dye removal such as methylene blue (MB). The in situ electrochemical spectroscopy suggests that the 3D rGO prefers to adsorb the reduced form of MB. For lithium-storage capability, the 3D rGO aerogel is ideal since it can be used as the high-energy anode of lithium batteries. It can provide a reversible specific capacity of over 600 mAh g-1 at 0.5 A g-1 and over 1000 mAh g-1 at 0.1 A g-1, which are much higher than a theoretical maximum value (372 mAh g-1) of graphite.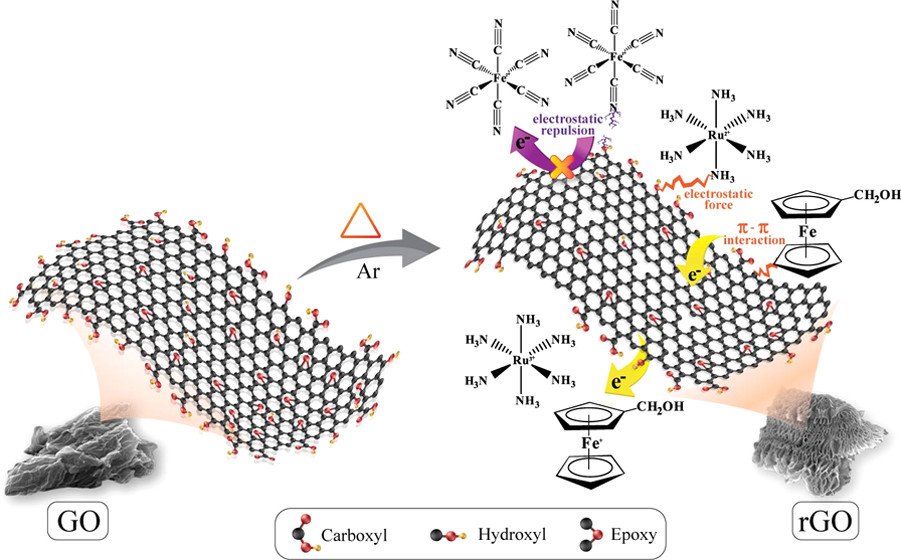

4. Synthesis of nickel hydroxide/delaminated-Ti3C2 MXene nanosheets as promising anode material for high performance lithium-ion battery
Li, C., Xue, Z., Qin, J., Sawangphruk, M., Yu, P., Zhang, X., Liu, R.
Journal of Alloys and Compounds, 2020, 842, 155812
DOI: https://www.sciencedirect.com/science/article/pii/S0925838820321769?via%3Dihub
Abstract: Elsevier B.V. In the present work, nickel hydroxide modified delaminated-Ti3C2 sheets (Ni(OH)2/d-Ti3C2) were synthetized by the hydrothermal method in order to overcome the capacity degradation caused by the terminal functional groups of Ti3C2-MXenes. Due to synergetic effects, the obtained Ni(OH)2/d-Ti3C2 displayed a capacity of 732.6 mA h g−1 at 0.1 A g−1, ∼6.0 times higher than that of d-Ti3C2 (∼121.3 mA h g−1), and ∼6.2 times higher than that of Ni(OH)2 (∼117.6 mA h g−1). Moreover, a higher reversible capacity of 372.0 mA h g−1 after 1000 cycles without apparent capacity decay at 1 A g−1 was obtained. This enhanced performance can be caused by the higher lithium ion diffusion coefficient in Ni(OH)2/d-Ti3C2 (2.5 × 10−14 cm2 s−1), ∼284.1 times higher than that of Ni(OH)2 (8.8 × 10−17 cm2 s−1) and ∼7.1 times higher than that of d-Ti3C2 (3.5 × 10−15 cm2 s−1). DFT calculations further confirmed that the Ni(OH)2/d-Ti3C2 can be a good candidate for the anode materials of lithium ion batteries.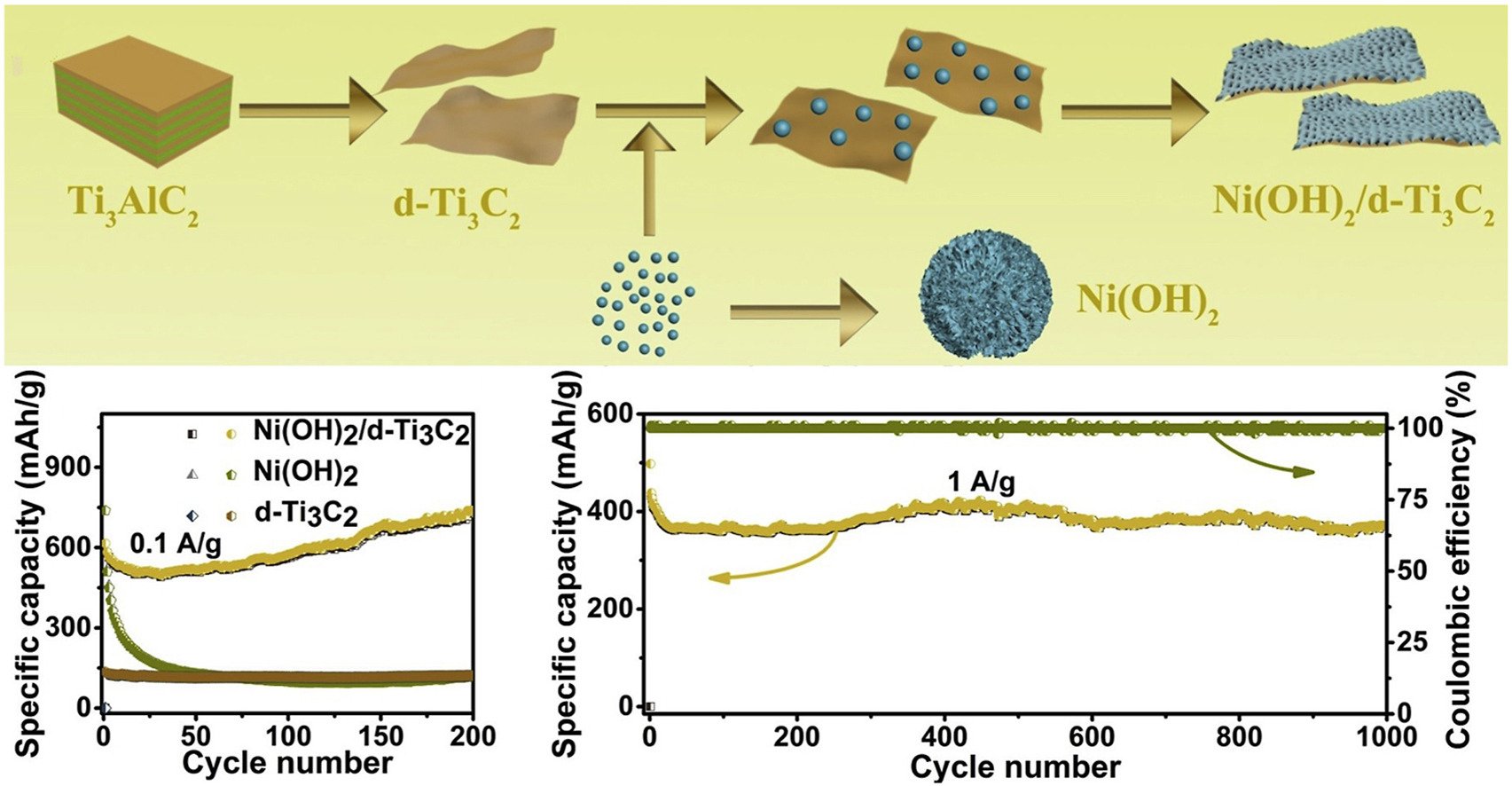

5. Effect of intercalants inside birnessite-type manganese oxide nanosheets for sensor applications
Wuamprakhon, P., Krittayavathananon, A., Kosasang, S., Ma, N., Maihom, T., Limtrakul, J., Chanlec, N., Kidkhunthod, P., Sawangphruk, M.
Inorganic Chemistry, 2020 59, 15595-15605
DOI: https://pubs.acs.org/doi/10.1021/acs.inorgchem.0c01592
Abstract: American Chemical Society. Hydrazine is a common reducing agent widely used in many industrial and chemical applications; however, its high toxicity causes severe human diseases even at low concentrations. To detect traces of hydrazine released into the environment, a robust sensor with high sensitivity and accuracy is required. An electrochemical sensor is favored for hydrazine detection owing to its ability to detect a small amount of hydrazine without derivatization. Here, we have investigated the electrocatalytic activity of layered birnessite manganese oxides (MnO2) with different intercalants (Li+, Na+, and K+) as the sensor for hydrazine detection. The birnessite MnO2 with Li+ as an intercalant (Li-Bir) displays a lower oxidation peak potential, indicating a catalytic activity higher than the activities of others. The standard heterogeneous electron transfer rate constant of hydrazine oxidation at the Li-Bir electrode is 1.09- and 1.17-fold faster than those at the Na-Bir and K-Bir electrodes, respectively. In addition, the number of electron transfers increases in the following order: K-Bir (0.11 mol) < Na-Bir (0.17 mol) < Li-Bir (0.55 mol). On the basis of the density functional theory calculation, the Li-Bir sensor can strongly stabilize the hydrazine molecule with a large adsorption energy (-0.92 eV), leading to high electrocatalytic activity. Li-Bir also shows the best hydrazine detection performance with the lowest limit of detection of 129 nM at a signal-to-noise ratio of ~3 and a linear range of 0.007-10 mM at a finely tuned rotation speed of 2000 rpm. Additionally, the Li-Bir sensor exhibits excellent sensitivity, which can be used to detect traces of hydrazine without any effect of interference at high concentrations and in real aqueous-based samples, demonstrating its practical sensing applications.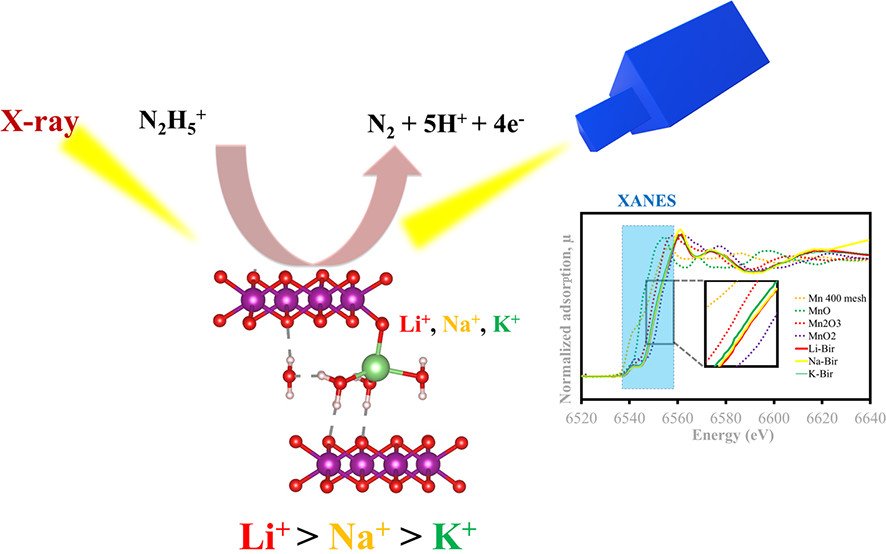

6. NiCo-LDH/Ti3C2 MXene hybrid materials for lithium ion battery with high-rate capability and long cycle life
Zhang, R., Xue, Z., Qin, J., Sawangphruk, M., Zhang, X., Liu, R.
Journal of Energy Chemistry, 2020, 50, 143-153
DOI: https://www.sciencedirect.com/science/article/pii/S2095495620301339?via%3Dihub
Abstract: Nickel/cobalt-layered double hydroxides (NiCo-LDH) have been attracted increasing interest in the applications of anode materials for lithium ion battery (LIB), but the low cycle stability and rate performance are still limited its practice applications. To achieve high performance LIB, the surface-confined strategy has been applied to design and fabricate a new anode material of NiCo-LDH nanosheet anchored on the surface of Ti3C2 MXene (NiCo-LDH/Ti3C2). The ultra-thin, bended and wrinkled α-phase crystal with an interlayer spacing of 8.1 Å can arrange on the conductive substrates Ti3C2 MXene directly, resulting in high electrolyte diffusion ability and low internal resistance. Furthermore, chemical bond interactions between the highly conductive Ti3C2 MXene and NiCo-LDH nanosheets can greatly increase the ion and electron transport and reduce the volume expansion of NiCo-LDH during Li ion intercalation. As expected, the discharge capacity of 562 mAh g−1 at 5.0 A g−1 for 800 cycles without degradation can be achieved, rate capability and cycle performance are better than that of NiCo-LDH (~100 mAh g−1). Furthermore, the density function theory (DFT) calculations were performed to demonstrate that NiCo-LDH/Ti3C2 system can be used as a highly desirable and promising anode material for lithium ion battery.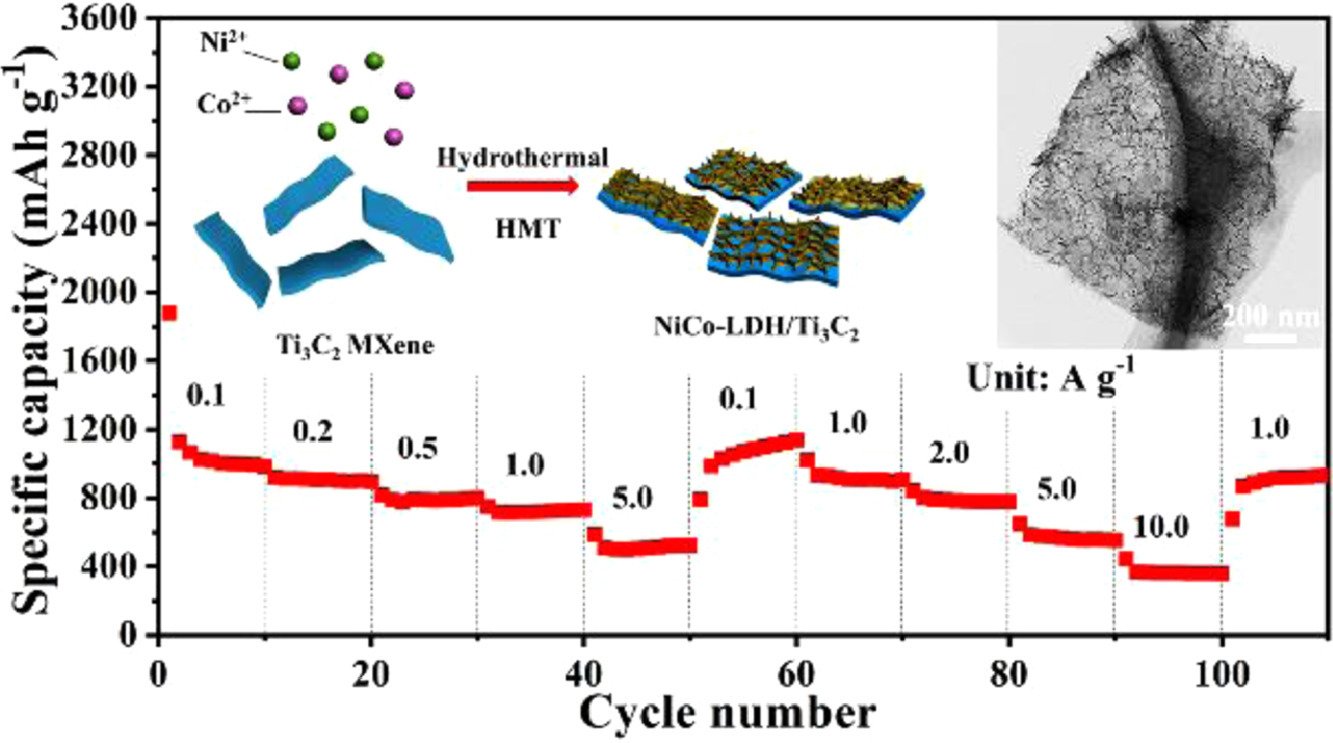

7. Prelithiated perfluoro-ionomer as an alternative binder for the state-of-the-art Ni-rich LiNi0.8Co0.15Al0.05O2 cathode of next-generation lithium-ion batteries
Vadivel, S., Sawangphruk, M.
Journal of Materials Chemistry A, 2020, 8, 20714-20724
DOI: https://pubs.rsc.org/en/content/articlelanding/2020/TA/D0TA04999A#!divAbstract
Abstract: A highly flexible and ionically conductive pre-lithiated perfluoro ionomer has been employed as a promising binder for the state-of-the-art Ni-rich (LiNi0.8Co0.15Al0.05O2, NCA) cathode. Such binder has been processed in two different solvent media concerning the solubility of surface residues (in ethanol and NMP), and their influence in electrochemical performances was compared. The binder dissolved in ethanol medium displays high initial coulombic efficiency in the formation cycle, followed by superior rate retention and cycling stability due to the significant solubility of lithium residues, when compared to the NMP mediated system. The operando XRD studies reveal that both systems undergo typical anisotropic crystallite alteration during the reversible lithium extraction; however, the ethanol-mediated electrode experiences a significantly low unit-cell volume change. The differential specific capacity studies also show that the electrode processed in ethanol is less prone to voltage decay, especially at the beginning of discharge, indicating improved lithium kinetics. In addition, the diffusion coefficient is found to be 5.69 × 10-9 cm2 s-1, related to the first anodic peak.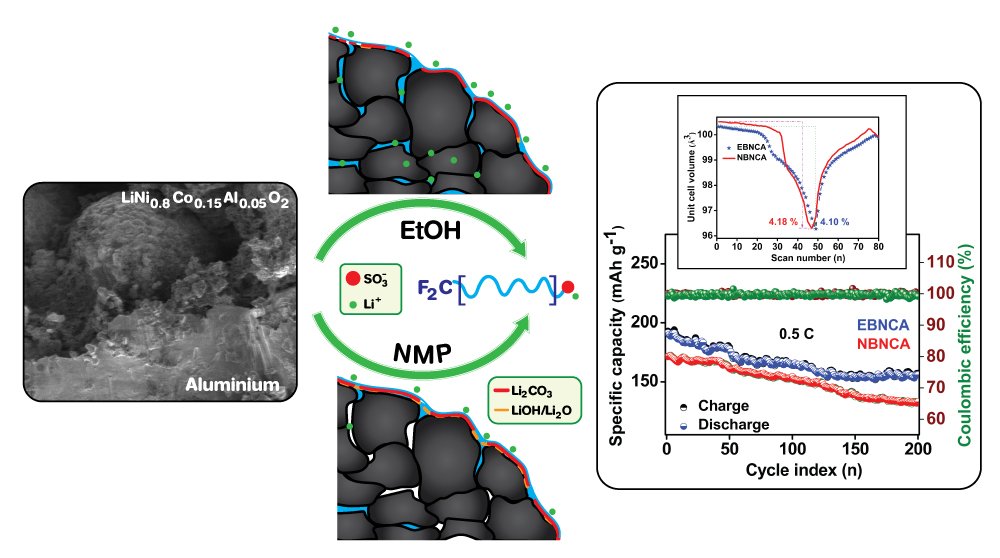

8. Scalable solvent-free mechanofusion and magnesiothermic reduction processes for obtaining carbon nanospheres-encapsulated crystalline silicon anode for Li-ion batteries
Wutthiprom, J., Phattharasupakun, N., Tomon, C., Sawangphruk, M.
Electrochimica Acta, 2020, 352, 136457
DOI: https://www.sciencedirect.com/science/article/pii/S0013468620308501?via%3Dihub
Abstract: Elsevier Ltd It is unquestionable that Si nanostructures i.e., nanosheets, nanowires, nanoparticles become more and more importance in high-energy lithium ion batteries (>300 Wh kg−1). However, the current commercial Si nanostructures are rather expensive, which is not yet able to complete with the graphite anode in term of USD/Wh kg−1. Herein, the conventional food grade non-porous silicon dioxide (SiO2) which costs 270-times lower than Si micron-sized and 3000-times lower than Si nano-sized (10 nm) is selected as a precursor for synthesizing pure crystalline Si nanoparticles. A novel scalable solvent-free mechanofusion method was firstly introduced to synthesize carbon nanospheres-encapsulated SiO2 (SiO2@C) followed by the reduction process producing silicon-carbon nanoparticles core-shell materials (Si@C) with interparticle void space. The carbon shell can prevent the volume expansion (>400%) of inner Si particles, which is a critical drawback of Si anode. In addition, the pre-lithiated Si@C anode obtained by a direct contact with Li counter electrode can address the poor coulombic efficiency at the first cycle. The pre-lithiated Si@C anode can deliver a reversible discharge specific capacity of 1390 mAh g−1 with a remarkable capacity retention of 90.4% and coulombic efficiency of ∼100% after 1000 cycles at a high rate of 1C. The ex situ TEM and XPS investigated confirm that the inner Si is well-confined within carbon buffer shell without being directly exposed to the electrolyte. Besides, an in operando XRD shows the reversible phase transformation during cycling for which Li15Si4 alloy is the product indicating that Si@C prepared in this work may be an ideal practical anode of high-energy Li-ion batteries.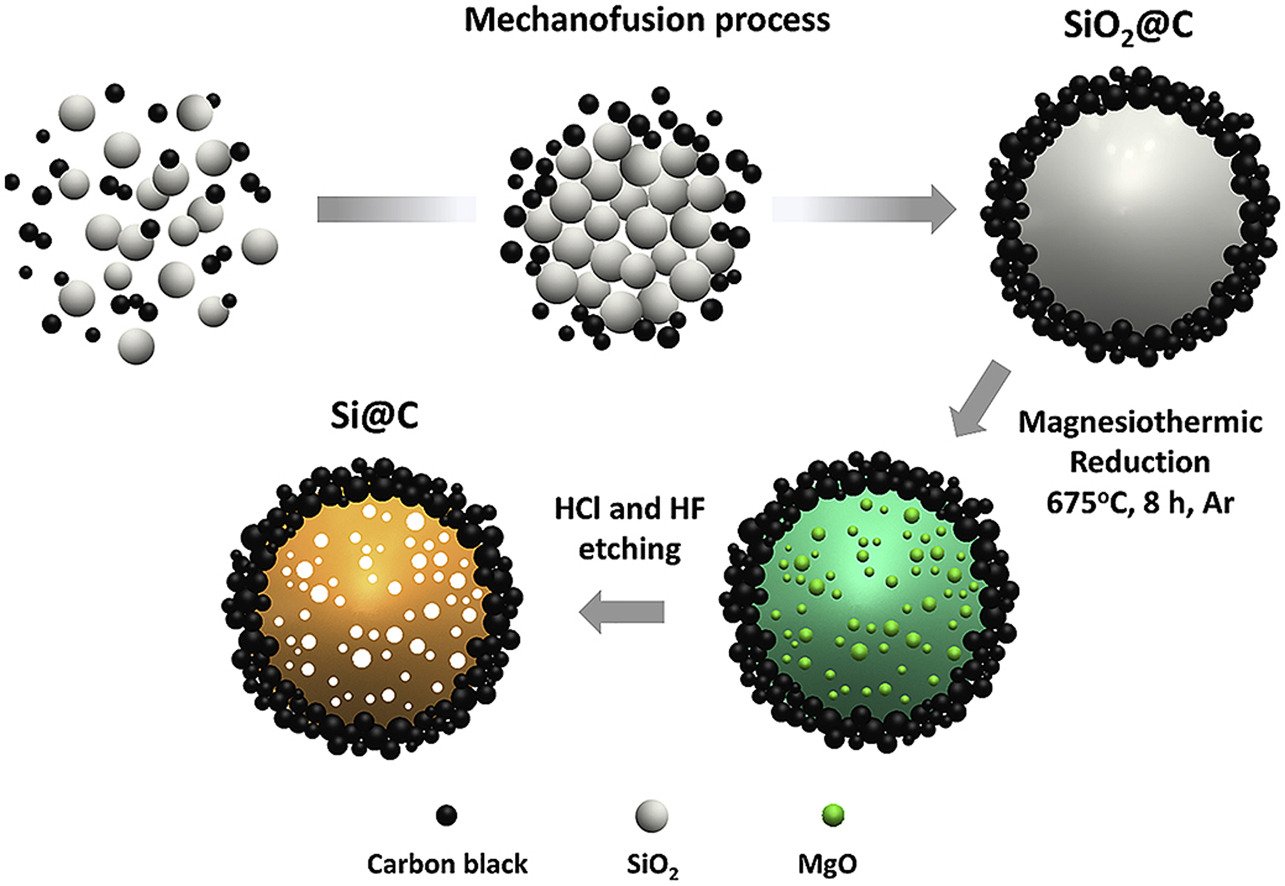

9. A Metal Organic Framework Derived Solid Electrolyte for Lithium–Sulfur Batteries
Chiochan, P., Yu, X., Sawangphruk, M., Manthiram, A.
Advanced Energy Material, 2020, 10, 2001285
DOI: https://onlinelibrary.wiley.com/doi/full/10.1002/aenm.202001285
Abstract: WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim Lithium–sulfur batteries (LSBs) are currently considered as promising candidates for next-generation energy storage technologies. However, their practical application is hindered by the critical issue of the polysulfide-shuttle. Herein, a metal organic framework (MOF)-derived solid electrolyte is presented to address it. The MOF solid electrolyte is developed based on a Universitetet i Oslo (UIO) structure. By grafting a lithium sulfonate (-SO3Li) group to the UIO ligand, both the ionic conductivity and the polysulfide-suppression capability of the resulting -SO3Li grafted UIO (UIOSLi) solid electrolyte are greatly improved. After integrating a Li-based ionic liquid (Li-IL), lithium bis(trifluoromethanesulfonyl)imide in 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide, the resulting Li-IL/UIOSLi solid electrolyte exhibits an ionic conductivity of 3.3 × 10−4 S cm−1 at room temperature. Based on its unique structure, the Li-IL/UIOSLi solid electrolyte effectively restrains the polysulfide shuttle and suppresses lithium dendritic growth. Lithium–sulfur cells with the Li-IL/UIOSLi solid electrolyte and a Li2S6 catholyte show stable cycling performance that preserves 84% of the initial capacity after 250 cycles with a capacity-fade rate of 0.06% per cycle.
10. Graphite/Graphene Composites from the Recovered Spent Zn/Carbon Primary Cell for the High-Performance Anode of Lithium-Ion Batteries
Vadivel, S., Tejangkura, W., Sawangphruk, M.
ACS Omega, 2020, 5, 15240-15246
DOI: https://pubs.acs.org/doi/10.1021/acsomega.0c01270
Abstract: American Chemical Society. Exploring electrochemically chapped graphite/graphene composites derived from the bulk carbon rod of the spent Zn/carbon primary cell is for the advanced high-capacity lithium-ion battery anode. It is found that the synthesized graphitic carbon has grain boundary defects with multilayered exfoliation. Such material exhibits an average specific capacity of 458 mA h g-1 at 0.2 C, which is higher than the theoretical specific capacity (372 mA h g-1) of graphite. The differential specific capacity calculations also show no significant difference in lithiation and delithiation potentials for the exfoliated sample at the low voltage. However, two additional plateaus have also been observed at ∼1.2 and 2.5 V, which confirms the formation of the LiC3 phase similar to lithiation of graphene. Hence, the superior lithiation ability and thecycling stability of defected graphite/graphene flakes may be useful for the sustainable development of next-generation high energy lithium-ion batteries. Also, waste recovery tends to reduce the risk of environmental pollution and the cost of raw materials.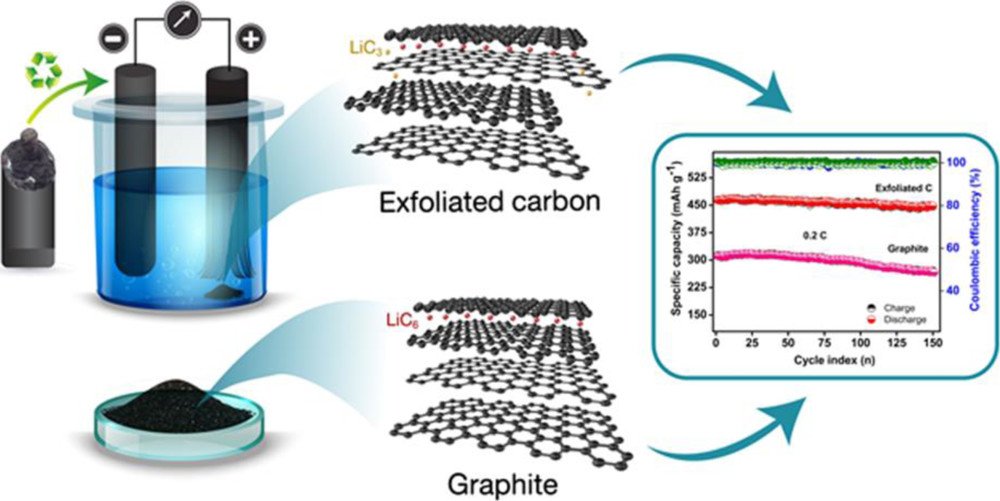

Sathyamoorthi, S., Tubtimkuna, S., Sawangphruk, M.
Journal of Energy Storage, 2020, 29, 101379
DOI: https://www.sciencedirect.com/science/article/pii/S2352152X2030236X?via%3Dihub
Abstract: Elsevier Ltd Achieving high cell potential supercapacitors in aqueous-based electrolytes is an essential step to the development of safe energy storage devices with high energy density. It is well known that the extension of cell potentials is due to the chemisorption of hydrogen in the micropores of activated carbon electrodes in neutral aqueous electrolytes. In this work, we have found that the structural feature, electronic property, and functional group of carbon electrodes do also play important roles to the cell potentials and performances of carbon-based supercapacitors. We employed four different carbons i.e., carbon fiber paper (CFP), functionalized carbon fiber paper (f-CFP), semiconducting macroporous reduced graphene oxide (rGO), and microporous activated carbon (AC) having different morphologies, structures, and functional groups. The symmetrical supercapacitors of AC and rGO show the maximum cell potential of 1.6 V by floating up to 200 h. This indicates that not only does the chemisorption of hydrogen within micropores play a key role on the wide working cell potentials (> 1.23 V) but others also play an important role confirmed by in situ mass spectrometry. This work may be useful for further development of carbon-based supercapacitors.

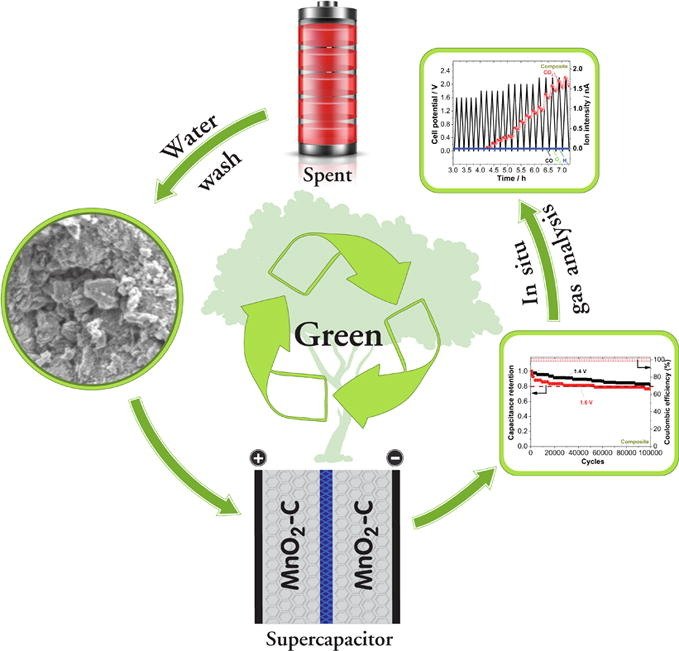
12. Turning carbon-ZnMn2O4 powder in primary battery waste to be an effective active material for long cycling life supercapacitors: In situ gas analysis
Sathyamoorthi, S., Tejangkura, W., Sawangphruk, M.
Waste Management, 2020, 109, 202-211
DOI: https://www.sciencedirect.com/science/article/abs/pii/S0956053X20302336?via%3Dihub
Abstract: Elsevier Ltd A simple and chemical-free recycled carbon-ZnMn2O4 powder (composite) from the spent Zn-carbon batteries is proposed as an effective active material for the supercapacitor. This approach may amplify the economic and environmental benefits of the recycling process. We also synthesized the spherical MnOx nanoparticles by the calcination followed by the chemical treatment. Recycled composite and the MnOx nanoparticles were comprehensively characterized. Symmetrical supercapacitors with the composite and the MnOx nanoparticles show specific capacitances of 118 F g−1 and 88 F g−1 at 0.1 A g−1, respectively. Also, the supercapacitor with the composite offers a specific energy of 8.0 Wh kg−1 at 0.1 A g−1 while 4.3 Wh kg−1 is obtained for MnOx nanoparticles. The stable cell potential limits of 1.4 V and 1.2 V were established for the supercapacitors of the composite and MnOx nanoparticles with the capacitance retention of 83% and 96%, respectively at the end of 100,000 cycles at 2.5 A g−1. Also, the excellent energy efficiencies of 80% and 72% with the coulombic efficiency of 100% are estimated for the supercapacitors of the composite and the MnOx nanoparticles, respectively. Finally, in situ gas analysis of the symmetrical supercapacitors are carried out using the differential electrochemical mass spectrometry. The proposed approach may be more economical and the environmentally benign recycling of spent Zn-carbon battery for circular economy and sustainability.

13. A universal and facile approach to suppress dendrite formation for a Zn and Li metal anode
Cao, J., Zhang, D., Zhang, X., Sawangphruk, M., Qin, J., Liu, R.
Journal of Materials Chemistry A, 2020, 8, 9331-9344
DOI: https://pubs.rsc.org/en/content/articlelanding/2020/TA/D0TA02486D#!divAbstract
Abstract: The Royal Society of Chemistry. To solve the poor cycling stability of zinc ion batteries (ZIBs) caused by the growth of zinc (Zn) dendrites, a novel method of separator modification is proposed. Herein, graphene oxide (GO), which has many outstanding properties, is applied as a modified material to a glass fiber (GF) separator using a simple vacuum filtration method. A stable and dendrite-free Zn anode can be obtained after 500 cycles in a Zn//Zn symmetrical battery when using a modified separator (GF/GO1), which is mainly owing to the preferential growth of the non-protruding crystal planes of the zinc metal and the uniform nucleation of zinc ions under the action of GO. As proof of concept, full Zn//MnO2 batteries with a GF/GO1 separator exhibited a high specific capacity of 126 mA h g-1 at 0.1 A g-1, a high energy density of 327.5 W h kg-1 (power density of 0.517 W kg-1), and a high power density of 20.8 kW kg-1 (energy density of 253.8 W h kg-1), demonstrating significant improvements compared with the unmodified GF separator. Meanwhile, ZIBs with a GF/GO1 separator exhibited excellent long-term stability with less than 25% capacity fade over 500 cycles at a current density of 0.5 A g-1. ZIBs with a GF separator retain only 37% of their initial capacity after 100 cycles. Furthermore, the GF/GO1 separator can also help to improve the cycle performance and rate performance of lithium metal batteries and to achieve a dendrite-free lithium anode. Full batteries of Li//GF/GO1//LiCoO2 show improved rate and cycling capabilities. A higher energy density of 537.1 W h kg-1 at 0.04 kW kg-1 and higher power density of 1.4 kW kg-1 at 264.6 W h kg-1 can be achieved with a GF/GO1 separator, which is better than many Li metal batteries. This modification of the separator provides an effective approach to designing next-generation batteries exhibiting excellent rate and cycling capabilities, and high energy and power densities.

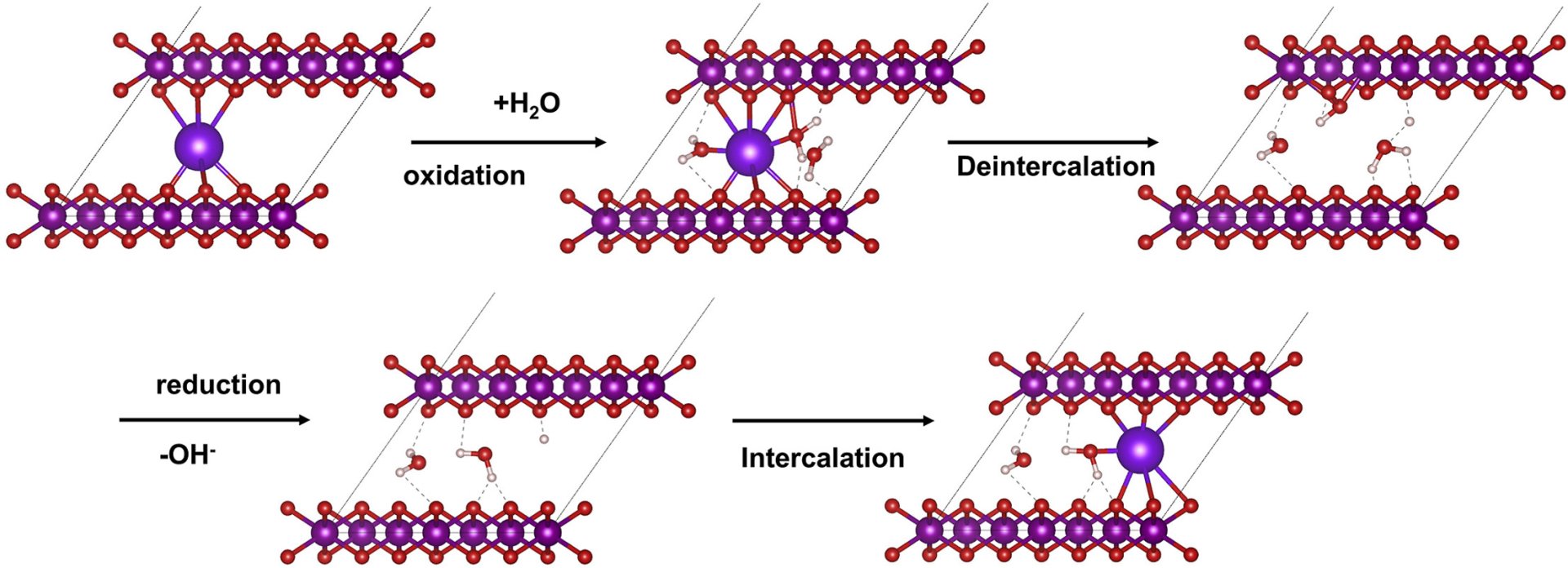
14. Insight into the unusual intercalation/deintercalation phenomena of alkali cations in the layered manganese oxide for electrochemical capacitors
Ma, N., Kosasang, S., Chomkhuntod, P., Duangdangchote, S., Phattharasupakun, N., Klysubun, W., Sawangphruk, M.
Journal of Power Sources, 2020, 455, 227969
DOI: https://www.sciencedirect.com/science/article/pii/S037877532030272X?via%3Dihub
Abstract: Elsevier B.V. The unusual intercalation phenomena of alkali cations (Li+, Na+, K+, Rb+, and Cs+) in the sulfate-based electrolyte on the electrochemical behavior of birnessite-type layered-manganese oxide nanosheets with Li-intercalated cation (Li-MnO2) were studied by in situ electrochemical synchrotron X-ray Absorption spectroscopy. Li-MnO2 exhibits the highest charge storage capacity in Na2SO4(aq), followed by Li2SO4, K2SO4, and Cs2SO4, respectively. Whilst, it does not exhibit the charge-storage characteristics in Rb2SO4 as the solvated Rb+ could not intercalate into the lamellar structure of birnessite. Understanding unusual intercalation phenomena can be useful for further development of the electrochemical capacitors.

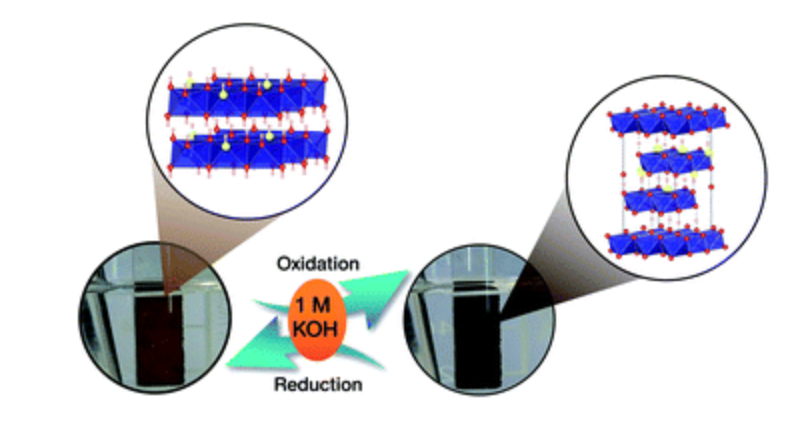
15. Cobalt oxysulphide/hydroxide nanosheets with dual properties based on electrochromism and a charge storage mechanism
Kalasina, S., Kongsawatvoragul, K., Phattharasupakun, N., Phattharaphuti, P., Sawangphruk, M.
RSC Advances, 2020, 10, 14154-14160
DOI: https://pubs.rsc.org/en/content/articlelanding/2020/RA/D0RA01714K#!divAbstract
Abstract: The Royal Society of Chemistry. The charge storage mechanism of mixed cobalt oxysulphide/hydroxide materials having electrochromic properties was investigated. The cobalt oxysulphide/hydroxide materials exhibit a dual reversible redox reaction and electrochromic properties in 1 M KOH during charging and discharging.

16. The Hybrid Energy Conversion and Storage of Nickel Metal Hydride (NiMH) Batteries
Kongsawatvoragul, K., Sawangphruk, M.
ECS Transactions, 2020, 97, 57-69
DOI: https://iopscience.iop.org/article/10.1149/09707.0057ecst
Abstract: The Electrochemical Society. Nickel metal hydride (NiMH) batteries are one type of batteries which are widely used commercially for various applications for example hybrid cars. NiMH battery consists of nickel hydroxide/oxyhydroxide (Ni(OH)2/NiOOH) cathode and lanthanum (La) alloy anode. Many recent studies focused on developing the storage capacity, the self-discharge, and the degradation of performance of NiMHs at a high temperature since they are cheaper than lithium-ion (Li-ion) batteries. Here, we have integrated the sunlight conversion and the storage abilities of Ni(OH)2 coated on transparent fluorene-doped tin oxide (FTO) glass for photoactive NiMH battery. This finding may lead to further develop novel photoactive NiMHs.

17. Improving Interfacial Contact within Solid-State Lithium Batteries using the Composite Materials at the Cathode Produced by a Scalable Mechanofusion Process
Bunyanidhi, P., Sawangphruk, M.
ECS Transactions, 2020, 97, 267-277
DOI: https://iopscience.iop.org/article/10.1149/09707.0267ecst
Abstract: The Electrochemical Society. Even though PEO-based electrolytes have been interested as a solution for developing the next-generation lithium-based batteries, their performances are still far from the commercial need and requirement. One of the major challenges limiting the cycling performance of all-solid-state batteries is at the interfacial contact of the electrode and the ceramic electrolyte, not like in the case of liquid electrolyte. To address this poor solid-solid contact, we introduce a cathode composite concept by combining both ceramic filler and polymer with LiFePO4 (LFP) cathode material. Herein, the garnet (Li7La3Zr2O12, LLZO) ceramic is well mixed with LFP cathode material using a new solid-solid mixing process namely a mechano-fusion technique. PEO is also used as a binder during the cathode preparation. The PEO-based composited electrolyte layer (filled with LLZO) and the composite material used at the cathode are stacked layer-by-layer and hot-pressed. As a result, this composite concept produced by a scalable mechanofusion process could be useful for further develop high-performance the all-solid-state batteries.

18. Advanced Hybrid 18650 Li-Ion Capacitors of Lithium Titanate (LTO)/Activated Carbon
Aphirakaramwong, C., Sawangphruk, M.
ECS Transactions, 2020, 97, 291-299
DOI: https://iopscience.iop.org/article/10.1149/09707.0291ecst
Abstract: The Electrochemical Society. There are remarkedly increasing demands in electronic devices including the portable electronic devices and electric vehicles (EVs). Therefore, the energy storages that can store energy efficiently with both high energy and power densities with good cyclability as display in batteries and supercapacitors are needed. The hybrid energy storages are one of the energy storage devices merging the advantages of both batteries and supercapacitors to overcome those lacking properties in each device. Thus, the lithium-ion hybrid capacitors (LICs) are introduced consisting battery-type as negative electrode and supercapacitors-type as positive electrode. There are many selections of electrode materials that can be used in the LICs such as carbonaceous materials and lithium titanate (LTO). The cell configuration was developed and fabricated in organic electrolyte since coin-cell to cylindrical cell. Herein, the 18650 configuration is introduced as hybrid energy storage consisting LTO and activated carbon as negative and positive electrodes, respectively. Moreover, the additional additives in electrolyte are also studied and investigated to improve the electrochemical performances to the hybrid capacitors.
19. Effect of cations in imidazolium-based ionic liquids on 18650 supercapacitors of activated carbon
Wuamprakhon, P., Donthongkwa, R., Sawangphruk, M.
ECS Transactions, 2020, 97, 13-24
DOI: https://iopscience.iop.org/article/10.1149/09707.0013ecst
Abstract: The Electrochemical Society The energy storage device with high energy and power densities with good cyclability is required. In principle, the energy density or specific energy of supercapacitor is proportional to the square of working potential window. In this work, different ionic liquids (ILs) with wide working potential windows are used as the electrolyte of supercapacitors using activated carbon. ILs of imidazolium-based structures with different sizes of alkyl chain (i.e., ethyl, propyl, butyl and pentyl) are synthesized to investigate their effects on the performance of supercapacitors. Also, we fabricated the 18650 cylindrical cells of symmetrical supercapacitors. The device with EMI-TFSI provides highest performance following by PMI-TFSI, BMI-TFSI and PTMI-TFSI, respectively. This device reveals the specific capacitance of 3.44 and 22.28 F/g at 100 mA as function of cell mass and active mass, respectively. Moreover, optimum specific energy of this device (3.30 Wh kg-1 and 21.33Wh kg-1 as function of cell mass and active mass, respectively) is higher than PMI-TFSI, BMI-TFSI and PTMI-TFSI around 15, 80 and 85 %, respectively. The results elucidate that the size of alkyl chain of imidazolium cation play the importance role in electrochemical of device. We hope that these results may pave a way to design the structure of IL electrolyte for high energy storage in large-scale production.
20. Graphite Layer Coated on Aluminium Foil as Anti-corrosion Current Collector for Neutral Aqueous Supercapacitors
Chomkhuntod, P., Sawangphruk, M., Kongsawatvoragul, K.
ECS Transactions, 2020, 97, 3-11
DOI: https://iopscience.iop.org/article/10.1149/09707.0003ecst
Abstract: The Electrochemical Society. Neutral aqueous supercapacitors are of interest due to their many advantages such as low-cost, non-flammable, and high ionic conductivity. Interestingly, the current collector is a critical part of these devices since it can be corroded in aqueous electrolytes. The Al foil is a widely used current collector for commercial organic supercapacitors due to its low-cost. However, it is corroded in aqueous electrolytes. To address this issue, we develop the graphite coating layer on the Al current collector to suppress the corrosion of Al and the formation of an oxide-resistive film on Al in an aqueous electrolyte. The corrosion testing was confirmed by the electrochemical impedance spectroscopy (EIS). Furthermore, the electrochemical performance of the as-prepared electrodes with and without the graphite coated layer was studied by using cyclic voltammetry (CV) and galvanostatic charge/discharge (GCD). The as-prepared electrode with a graphite protective layer exhibits a higher specific capacitance as well as a higher rate capability. The capacitance fading upon increasing scan rate from 10 mV s-1 to 100 mV s-1 of graphite coated aluminium electrode was only 15%, while the capacitance of the unmodified electrode extremely faded up to 75%. The as-prepared electrode with a graphite protective layer can explicitly improve the cycling stability (retain 90 %) after 5,000 cycles at an applied current density of 1 A g-1, which is different from an unmodified electrode with rapid fading up to 55%. This finding may lead to practical neutral aqueous supercapacitors.
21. High-Performance 18650 Lithium-Ion Batteries Using Ni-Rich Cathode and Silicon Nanoparticles/MCMB Composites
Amnuaymucha, N., Duriyasart, F., Sawangphruk, M.
ECS Meeting Abstracts, 2020, MA2020-01, 466
DOI: https://iopscience.iop.org/article/10.1149/MA2020-012466mtgabs
Abstract: The Electrochemical Society. Silicon nanoparticles and MCMB (MesoCarbon MicroBeads) materials as well as their composites are used as anode materials of lithium-ion batteries with Ni-rich cathode materials. The optimum particle size and loading content of silicon nanoparticles in the composite are presented. The cylindrical lithium-ion batteries (18650) were fabricated in the dry room condition with a dew point of -40 °C. The stable structure of carbonaceous materials can alleviate the expansion problem of silicon by supporting silicon nanoparticles on its excellent conductive surface and structure. Therefore, the silicon-MCMB composite is a promising material for use as anode in Li-ion batteries. In this work, the silicon nanoparticles with < 10 nm in diameter exhibit high charge-storage performance. The NCA//Silicon-MCMB shows the first cycle capacity of 2.65 Ah per cell with an energy density of > 250 Wh/kg, an initial columbic efficiency of > 90%.
22. Effect of Electrolyte Additives on Cycling Performance of 18650 Graphite//NMC811 Li-ion Batteries
Tubtimkuna, S., Sawangphruk, M., Duriyasart, F.
ECS Transactions, 2020, 97, 155-166
DOI: https://iopscience.iop.org/article/10.1149/09707.0155ecst
Abstract: The Electrochemical Society. The practical application of Nickel-rich NMC811lithium-ion battery cylindrical cell type 18650 has been limited with its poor cycling stability due to transition metal dissolution, microcrack of NMC811 cathode, and thermodynamic instability of graphite anode. Herein, we study the effect of each electrolyte additive; VC, VEC, FEC, and PS, on the stability performance of the battery by varying the additive content at 1%, 2% and 3%. The study revealed the effect of electrolyte additives on the creation of stable SEI and CEI layers, which efficiently protected the surface of anode and cathode, respectively. It was found that high capacity retention of 61.98% can be obtained after 70 cycles, when 3%wt of VC, VEC, FEC, and PS was added. The average degradation rate of 0.54% per cycle was also observed. The improvement of cycling performance of the battery is confirmed.
23. Machine Learning and Reactive Force Field Molecular Dynamics Investigation of Electrolytes for Ultra-fast Charging Li-ion Batteries
Joraleechanchai, N., Duangdangchote, S., Sawangphruk, M.
ECS Transactions, 2020, 97, 45-55
DOI: https://iopscience.iop.org/article/10.1149/09707.0045ecst
Abstract: The Electrochemical Society. The ultra-fast charging batteries have become a bigger demand for electric vehicles. To achieve the fast charging system, the electrolyte system should be optimized with a high balance between ionic conductivity and chemical stability. In recent research, many compounds, including organic ester, formate, and nitrile, were introduced into carbonate-based electrolytes as a co-solvent. However, the understanding of how co-solvents and effect of functional groups improve the electrolyte properties is not yet disclosed. Here we investigate the role of co-solvent that affects electrolyte properties, especially the self-diffusion coefficient by using theoretical reactive molecular dynamics (RMDs) simulations coupled with the advancement in numerical method to compute the correlation between co-solvent properties and the self-diffusion coefficient. We found that the low electron affinity co-solvent and low-density molecule are the keys to improve the Li-ion diffusion in the carbonate-based electrolyte system. Hence, this work could lead the way for developing the new co-solvent for the ultra-fast charging demand.
24. Influence of Electrode Density on the Microstructural NCA Positive Electrode for Scalable 18650 Li-Ion Batteries
Sarawutanukul, S., Tomon, C., Phattharasupakun, N., Sawangphruk, M.
ECS Transactions, 2020, 97, 143-154
DOI: https://iopscience.iop.org/article/10.1149/09707.0143ecst
Abstract: The Electrochemical Society. Calendaring process is one of the most important part in the line production of 18650 lithium ion battery which densifies the electrode and promotes consistency to the metal current corrector. However, this process promotes the tortuosity of the void space network in the primary ion transportation and facilitate tradeoffs between electrode density and rate capability. The aim of this work to understanding the impact of density and porosity on electrode kinetics and authorizing cell designs with denser electrodes to improve capacity and energy density, this study investigates the effect of high and low limit of pressure force in the calendaring process between 400 and 2000 kg cm2 on the electrode properties such as density and porosity of LiNi0.8Co0.15Al0.05O2 (NCA) electrode. Its found that as the applied pressure force increased, the qualitative properties of NCA electrode are increased via the particle packing, porosity, and density. These factors directly affect to Li-ion diffusion in electrode and Li-ion depletion in the electrolyte interphases which will improve the energy density and power density. However, the overpressure force leads to increase in micro-cracking inside the NCA particle, tortuosity and over-packed of the NCA electrode which can obstructed the electrode capacity at high C-rates. Based on this result, the low-level compaction was proposed to mitigate the limiting factors. The development of the energy-power density in an 18650 cell was used to demonstrate how to further study into the electrode parameters in cell engineering.
25. The Protection of Lithium Metal Enabled by LiNO3 for Lithium-Sulfur Batteries
Duangdangchote, S., Krittayavathananon, A., Phattharasupakun, N., Sawangphruk, M.
ECS Transactions,2020, 97, 827-834
DOI: https://iopscience.iop.org/article/10.1149/09707.0827ecst
Abstract: The Electrochemical Society. An insight into the role of LiNO3 additive in an ether-based electrolyte for lithium sulfur battery has been presented. Herein, we proposed a formation mechanism of solid-electrolyte interphase on the surface of lithium metal anode by using the theoretical reactive force field (ReaxFF) simulation method. The interaction between the reactive lithium metal and nitrate ions results in the formation of LixNOy clusters, distributed homogeneously in both the SEI layer and electrolyte phase. Not only can these clusters be an efficient surface protection layer of lithium metal anode but also, they can suppress the lithium polysulfides shuttle effect by adsorbing liquid lithium polysulfide intermediates.
26. MnCo2O4 Nanofibers as Efficient Photo-electrocatalyst for Oxygen Evolution Reaction and Oxygen Reduction Reaction
Tomon, C., Sarawutanukul, S., Krittayavathananon, A., Sawangphruk, M.
ECS Transactions,2020, 97, 71-86
DOI: https://iopscience.iop.org/article/10.1149/09707.0071ecst
Abstract: The Electrochemical Society. Photoactive bimetal oxide catalysts towards oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) activities have not yet been comprehensively investigated. Herein, this work presents the MnCo2O4 nanofiber using as a photo-electrocatalyst for OER and ORR. After irradiation with visible light, the MnCo2O4 decreases ca. 17.1% and 15.4% in overpotential of OER and ORR, respectively, while the overpotential of the Co3O4 is reduced ca. 8.6% and 6.4% towards OER and ORR processes, respectively. These results suggest that MnCo2O4 can enhance OER/ORR performances with two-fold higher than Co3O4 under the light illumination. Due to the mixed valence states of Mn2 + and Mn3 + in its spinel structure, the MnCo2O4 has lower band gap energy (1.7 eV) than the Co3O4 nanofiber (2.0 eV). This narrower band gap can facilitate electron transfer from the valence band to the conduction band. Moreover, the photogenerated MnCo2O4 ∗ holes can accelerate the reaction with OH- and H2O which subsequently enhances OER performance.
27. Electrochemical Reduction of Carbon Dioxide Using CVD Graphene on Non-noble Metal Foams as Carbo-/Electro-Catalysts
Srimanon, K., Krittayavathananon, A., Sarawutanukul, S., Sawangphruk, M., Duangdangchote, S.
ECS Transactions, 2020, 97, 301-308
DOI: https://iopscience.iop.org/article/10.1149/09707.0301ecst
Abstract: The Electrochemical Society. Herein, we present the catalytic activity of CVD graphene on copper and nickel foam substrates towards electrochemical CO2 reduction (CO2R). As the result, the graphene on nickel foam (GP/NiF) shows the lower onset potential (-0.6 V vs. RHE) of CO2R process in 0.1 M KHCO3 under sat. CO2 atmosphere than that of copper foam (GP/CuF), -1.0 V vs RHE. When increasing the concentrations of KHCO3 from 0.1 to 2.0 M, the current density of the GP/NiF electrode increases due to the reduction of H+ or increase of pH.
28. Confining Li2S6 catholyte in 3D graphene sponge with ultrahigh total pore volume and oxygen-containing groups for lithium-sulfur batteries
Chiochan, P., Kosasang, S., Ma, N., Duangdangchote, S., Suktha, P., Sawangphruk, M.
Carbon Volume,2020, 158, 244-255
DOI: https://www.sciencedirect.com/science/article/pii/S0008622319312503?via%3Dihub
Abstract: Elsevier Ltd A three-dimensional reduced graphene oxide (3D rGO) sponge with an ultra-high specific pore volume of 6.4 cm3g-1 and oxygen-containing (e.g., carboxyl) groups finely tuned was designed, synthesized, and employed as an ideal host of Li2S6 catholyte and related compounds for high-performance lithium-sulfur batteries (LSBs). To the best of our knowledge, the 3D rGO in this work exhibits the highest specific pore volume ever. The interconnected porous structure of 3D rGO can totally confine the lithium polysulfides overcoming the shuttle effect. Also, it can offer an excellent electrical conductivity and electrolyte transportation leading to high charge storage capacity. The as-fabricated LSB provides a high discharge capacity of 1607 mAh g−1 at 0.1C and a high areal capacity of 3.53 mAh cm−2. Even at a high sulfur loading content of 6.6 mg cm−2, high utilization (79.4%) of active materials at the 0.1C and low capacity fading rate of 0.065% per cycle at the 1.0C with 98% coulombic efficiency over 200 cycles are achieved. The 3D rGO sponge could be useful for high-energy battery applications.

29. Elucidating the unexpected electrocatalytic activity of nanoscale PdO layers on Pd electrocatalysts towards ethanol oxidation in a basic solution
Krittayavathananon, A., Duangdangchote, S., Pannopard, P., Chanlek, N., Sathyamoorthi, S., Limtrakul, J., Sawangphruk, M.
Sustainable Energy and Fuels, 2020, 4, 1118-1125
DOI: https://pubs.rsc.org/en/content/articlelanding/2020/SE/C9SE00848A#!divAbstract
Abstract: Pd-based catalysts are typically used in many applications; however, the effect of their oxide layer has not been fully investigated to date. Herein, by using experimental and theoretical approaches, we have found that the oxide layer significantly affects the electrocatalytic activity of Pd towards the ethanol oxidation reaction (EOR) in a basic solution. It alters the surface morphology and active sites of the catalyst, leading to different electrochemical kinetics and mechanistic pathways. Nanoscale PdO(101) layers on Pd(111) with the thickness of a few nanometers can strongly bind with ethanol and its intermediate species (i.e., acetaldehyde and acetic acid), leading to high current density being observed electrochemically. Also, the high surface roughness and active sites of the nanoscale PdO(101) layer can stabilize adsorbed intermediates, resulting in high autocatalytic decomposition and leading to overall productivity. This is an expected result since bulk PdO(101) is rather poor in terms of catalytic activity and productivity. Tuning the surface chemistry of metal catalysts with nanoscale oxide layers is a critical process in improving the electrocatalytic activity and productivity.
30. The Influence of Hydration Energy on Alkali-Earth Intercalated Layered Manganese Oxides as Electrochemical Capacitors
Chomkhuntod, P., Ma, N., Kosasang, S., Duangdangchote, S., Phattharasupakun, N., Jangsan, C., Sawangphruk, M.
ACS Applied Energy Materials, 2020, 3, 1402–1409
DOI: https://pubs.acs.org/doi/10.1021/acsaem.9b01822
Abstract: Insight into the influence of hydration energy of structural cations within birnessite-type layered MnO2 on charge storage mechanisms via redox reaction and intercalation/deintercalation processes with the ion-exchange process is demonstrated. The redox activity and Mn utilization observed from ex situ X-ray absorption spectroscopy are Li-MnOx > Ca-MnOx > Sr-MnOx > Ba-MnOx. Although Li-MnOx shows higher redox activity than Ca-MnOx, the Ca-MnOx exhibits higher specific capacitance due to its higher hydration energy of Ca2+ (-500 kcal mol-1) as compared to -465 and -436 kcal mol-1 of Sr-MnOx and Ba-MnOx, respectively, which dominates the ion-exchange affinity within the birnessite structure. Therefore, the charge storage mechanism of the birnessite depends strongly on the hydration energy of structural cations which can further be probed by inductively coupled plasma-optical emission spectrometry technique. Additionally, Ca-MnOx with the smallest number of the remaining structural Ca2+ as compared with other cations demonstrates the highest specific capacitance followed by Sr-MnOx, Li-MnOx, and Ba-MnOx. Furthermore, all the as-prepared samples demonstrate the excellent cycling stability (above 96%) after 11 000 cycles at a current density of 5 A g-1. This finding may be useful for further development of practical manganese oxide supercapacitors.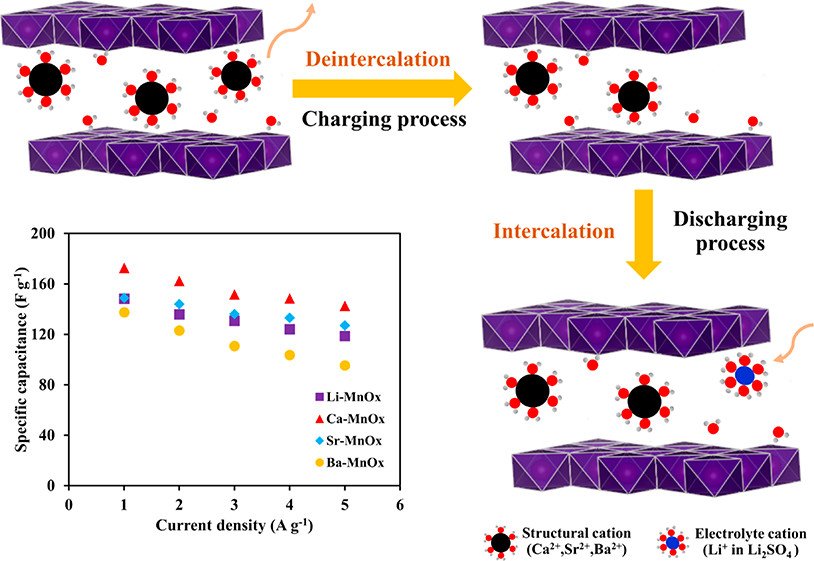

31. Impact of Al Doping and Surface Coating on the Electrochemical Performances of Li-Rich Mn-Rich Li1.11Ni0.33Mn0.56O2 Positive Electrode Material
Phattharasupakun, N., Geng, C., Johnson, M.B., Vali, R., Liu, A., Liu, Y., Sawangphruk, M., Dahn, J.R.
Journal of the Electrochemical Society, 2020, 167, 120531
DOI: https://iopscience.iop.org/article/10.1149/1945-7111/abb282
Abstract: Published on behalf of The Electrochemical Society by IOP Publishing Limited. Li and Mn-rich positive electrode materials, Li[LixTM1-x]O2 (TM = Ni, Co, and Mn), with a single-phase layered structure have been considered for use in next-generation Li-ion batteries for electric vehicles and many advanced applications. Despite their high specific capacity >250 mAh g-1, the commercialization of these materials is hindered by poor rate capability and voltage decay originating from transition metal migration to the lithium layer. Herein, the effect of aluminum doping and aluminum oxide surface coating on the structural and electrochemical performances of Co-free Li1.11Ni0.33Mn0.56O2 was studied. All synthesized materials were single phase with a similar morphology and amount of Ni in the Li layers. Even though the discharge capacity and capacity retention were slightly improved, there was no significant impact of the addition of Al on the rate of voltage fading during charge-discharge cycling as previously reported.
32. Single-atoms supported (Fe, Co, Ni, Cu) on graphitic carbon nitride for CO2 adsorption and hydrogenation to formic acid: First-principles insights
Homlamai, K., Maihom, T., Choomwattana, S., Sawangphruk, M., Limtrakul, J.
Applied Surface Science, 2020, 499, 143928
DOI: https://www.sciencedirect.com/science/article/pii/S0169433219327448?via%3Dihub
Abstract: Elsevier B.V. The non-noble metal single-atom catalysts (SACs) of Fe, Co, Ni and Cu supported on graphitic carbon nitride (g-C3N4) for CO2 adsorption and hydrogenation to formic acid have been investigated with periodic density functional theory calculations. From our calculations, we found the adsorption energies of CO2 in the range of −0.16 to −0.40 eV with the highest stability over Fe-g-C3N4. The van der Waals interaction was included in the calculation due to its significant role in CO2 adsorption. The 2-step proposed reaction mechanism involves the CO2 hydrogenation to form a formate intermediate and hydrogen abstraction to formic acid as the end product. Based on the rate-determining step activation barrier, the catalytic activity order was found as Fe-g-C3N4 > Co-g-C3N4 > Cu-g-C3N4 > Ni-g-C3N4. From our findings, the better understanding of the effect of the non-noble metal coordination on CO2 adsorption and hydrogenation provides hints to the rational catalyst design.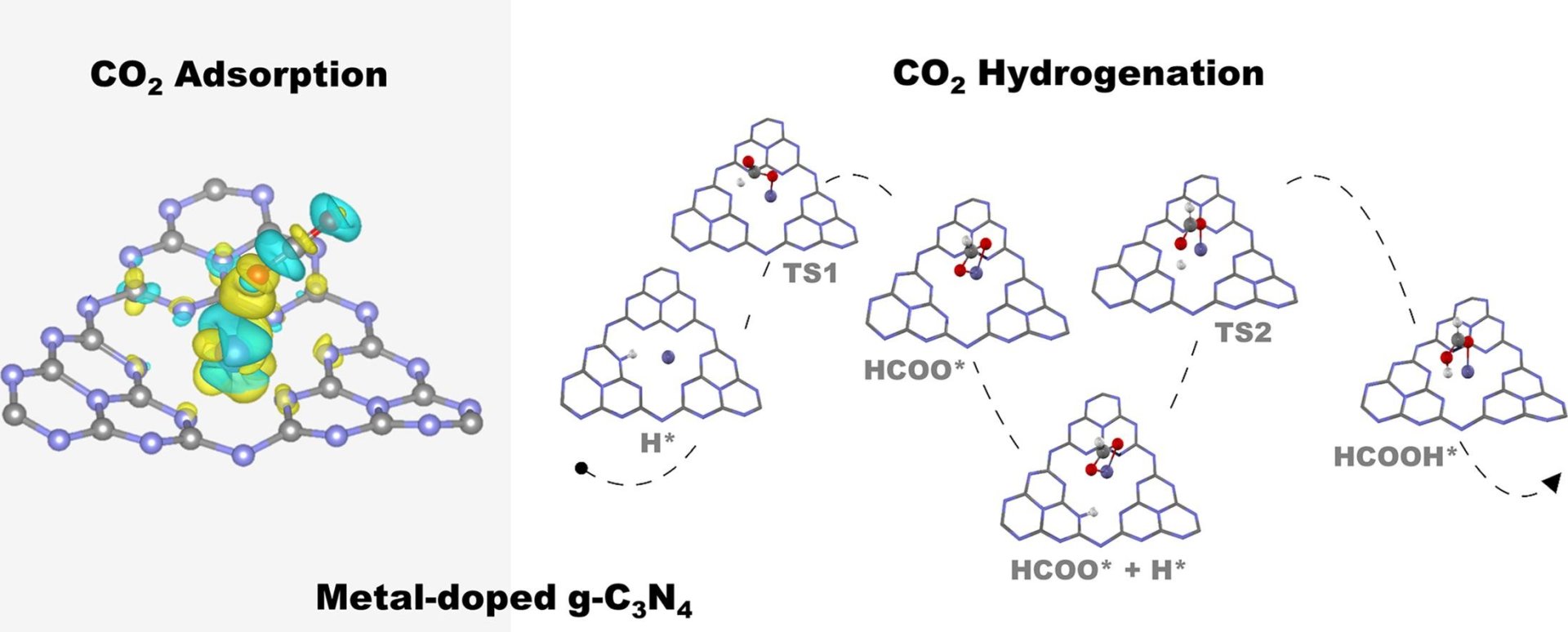

33. First Scalable 18650 Aqueous-based Supercapacitors Using Hydrophobicity of Anti-corrosion Graphite Passivation Layer
P Chomkhuntod, P Iamprasertkun, M Sawangphruk
Scientifc Reports, 2021. 11, 13082
DOI: https://www.nature.com/articles/s41598-021-92597-y
Abstract: Scalable 18650 aqueous-based supercapacitors are ideal as future energy storage technology due to their great safety, low cost, and environmental friendliness as well as high power density. Until now, there are no commercial aqueous-based supercapacitors due to the corrosion of metal current collectors. In this work, we have introduced a new concept using hydrophobicity of anti-corrosion graphite passivation layer coated on Al foil with high surface roughness leading to the lotus effect.
34. Controlling the flake size of bifunctional 2D WSe2 nanosheets as flexible binders and supercapacitor materials
Pawin Iamprasertkun, Wisit Hirunpinyopas, Varisara Deerattrakul, Montree Sawangphruk, Chakrit Nualchimplee
Nanoscale Advances, 2021, 3, 653-660
DOI: https://pubs.rsc.org/az/content/articlehtml/2020/na/d0na00592d
Abstract: A new approach using graphene as a conductive binder in electrical supercapacitors has recently been proposed. Graphene shows outstanding properties as a conductive binder, and can be used to replace conductive, additive, and polymer binders. However, graphene follows an EDLC behaviour, which may limit its electrochemical performance. In the process described in this work, we introduced WSe2 nanoflakes as a new approach to using pseudocapacitive materials as binders. The WSe2 nanoflakes were produced through liquid phase exfoliation of bulk WSe2, and the flake size was finely selected using a controlled centrifugation speed. The physical and electrochemical properties of the exfoliated WSe2 flakes were analysed; it was found that the smallest flakes (an average flake size of 106 nm) showed outstanding electrochemical properties, expanding our understanding of transition metal dichalcogenide (TMD) materials, and we were able to demonstrate the applicability of using WSe2 as a binder in supercapacitor electrodes. We also successfully replaced conductive additives and polymer binders with WSe2. The overall performance was improved: capacitance was enhanced by 35%, charge transfer resistance reduced by 73%, and self-discharge potential improved by 9%. This study provides an alternative application of using TMD materials as pseudo capacitive binders, which should lead to the continued development of energy storage technology.
35. Trimetallic Spinel-Type Cobalt Nickel-doped Manganese Oxides as Bifunctional Electrocatalysts for Zn-air Batteries
Soracha Kosasang, Harnchana Gatemala, Nattapol Ma, Praeploy Chomkhuntod, Montree Sawangphruk
Batteries & Supercaps, 2020
DOI: https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/batt.202000006
Abstract: This work demonstrates the effect of trimetallic and bimetallic electrocatalysts of spinel‐type metal oxides towards oxygen reduction reaction (ORR) and oxygen evolution reaction (OER). The trimetallic spinel‐type Co0.5Ni0.5Mn2O4 shows higher bifunctional electrocatalytic activity towards ORR and OER than bimetallic oxides such as CoMn2O4 and NiMn2O4. The in situ X‐ray absorption spectroscopy was applied to observe the change in the oxidation number of Mn, Ni, and Co during the reactions to demonstrate the active metals on the ORR and OER. The Co plays a more important role in the ORR process than the Ni and Mn, while the three metals exhibit an equivalent contribution on the OER activity as observed from the oxidation state shift. Additionally, the practicality of Zn‐air batteries using the trimetallic spinel catalyst is demonstrated to power light‐emitting diode and spinning motor with a nominal voltage of 3 V. Bifunctional trimetallic electrocatalysts in this work may be useful for other metal‐air batteries.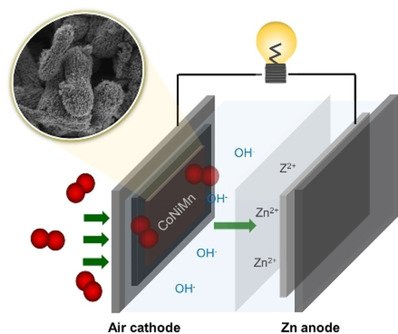

36. Rechargeable Photoactive Zn-air batteries Using NiCo2S4 as an Efficient Bifunctional Photocatalyst towards OER/ORR at the Cathode
Sangchai Sarawutanukul, Chanikarn Tomon, Salatan Duangdangchote, Phattharasupakun Nutthaphon, Montree Sawangphruk
Batteries & Supercaps. 2020
DOI: https://chemistry-europe.onlinelibrary.wiley.com/doi/epdf/10.1002/batt.201900205
Abstract: Using free solar energy in oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) electrocatalysts to enhance the efficiency of zinc‐air batteries (ZABs) has not yet been widely investigated. Herein, we report a photoactive bifunctional catalyst of spinel‐type NiCo2S4 (NCS) urchin‐like structure with rich mesopores and direct band gap energies of ca. 1.4 and 2.4 eV. The NCS catalyst exhibits high catalytic activities for both OER (ɳ=338 mV at 10 mA cm−2) and ORR (ɳ=475 mV at E1/2), comparable to that of the state‐of‐the‐art counterparts (e. g., Pt/C for ORR, RuO2 for OER). Under light illumination, the p‐type photoactive NCS catalyst can absorb visible light, generating photogenerated holes and photoelectrons via the photoelectric effect for direct conversion of photoenergy into electric energy with increasing kinetics charge transfer process and provides ca. 10 and 18.5 % lower OER and ORR overpotentials, respectively than those under the dark condition. In addition, the as‐fabricated zinc‐air battery with the photoactive NCS as the cathode exhibits decrease in voltage gap from 0.82 to 0.60 V with an increasing round‐trip efficiency from 59.2 % to 68.8 % after exposed to visible light. The zinc‐air battery with a reversible redox reaction for the simultaneous conversion of chemical and photoenergy into electric energy could open a new pathway for the utilization of a single energy conversion and storage device.